

���� ��1����B���ܷ���������Ӧ����˵����ˮ�����A��-OH���ڵ�̼ԭ����ֻ��1����ԭ�ӣ���AΪ2-����������Ϊ2-����飬BΪ��ͪ��CΪ2-��������ȥ���Ϊ��ϩ��DΪ�۱�ϩ���ݴ˷�����
��2����B���ܷ���������Ӧ����˵����ˮ�����A��-OH���ڵ�̼ԭ������2����ԭ�ӣ���AΪ1-����������Ϊ1-����飬BΪ��ȩ��CΪ1-��������ȥ���Ϊ��ϩ��DΪ�۱�ϩ���ݴ˷�����
��� �⣺��1����B���ܷ���������Ӧ����˵����ˮ�����A��-OH���ڵ�̼ԭ����ֻ��1����ԭ�ӣ���AΪ2-����������Ϊ2-����飬BΪ��ͪ��CΪ2-��������ȥ���Ϊ��ϩ��DΪ�۱�ϩ��
��AΪ2-�������ṹ��ʽΪ��CH3CH��OH��CH3���ʴ�Ϊ��CH3CH��OH��CH3��
��Aת��ΪBΪ���Ĵ���������ѧ����ʽΪ��2CH3CH��OH��CH3+O2$��_{��}^{Cu}$2CH3COCH3+2H2O���ʴ�Ϊ��2CH3CH��OH��CH3+O2$��_{��}^{Cu}$2CH3COCH3+2H2O��
���������ƵĴ���Һ��������±��������ȥ��Ӧ����ѧ����ʽΪ��CH3CHBrCH3+NaOH$��_{��}^{��}$CH2=CHCH3+NaBr+H2O���ʴ�Ϊ��CH3CHBrCH3+NaOH$��_{��}^{��}$CH2=CHCH3+NaBr+H2O��
��2����B���ܷ���������Ӧ����˵����ˮ�����A��-OH���ڵ�̼ԭ������2����ԭ�ӣ���AΪ1-����������Ϊ1-����飬BΪ��ȩ��CΪ1-��������ȥ���Ϊ��ϩ��DΪ�۱�ϩ��
��AΪ1-���������л���A�Ľṹ��ʽΪCH3CH2CH2OH���ʴ�Ϊ��CH3CH2CH2OH��
��BΪ��ȩ���ܺ�������ҺAg��NH3��2OH����������Ӧ����ѧ����ʽΪ��CH3CH2CHO+2Ag��NH3��2OH$\stackrel{��}{��}$H2O+2Ag+3NH3+CH3CH2COONH4���ʴ�Ϊ��CH3CH2CHO+2Ag��NH3��2OH$\stackrel{��}{��}$H2O+2Ag+3NH3+CH3CH2COONH4��
��ת��ΪC����1-����鷢��±��������ȥ��Ӧ���ʻ�ѧ����ʽΪ��CH3CH2CH2Br+NaOH$��_{��}^{�Ҵ�}$CH2=CHCH3+NaBr+H2O���ʴ�Ϊ��CH3CH2CH2Br+NaOH$��_{��}^{�Ҵ�}$CH2=CHCH3+NaBr+H2O��
BΪ��ȩ����������Cu��OH��2��Һ�ķ�Ӧ��CH3CH2CHO+NaOH+2Cu��OH��2$\stackrel{��}{��}$CH3CH2COONa+Cu2O��+3H2O���ʴ�Ϊ��CH3CH2CHO+NaOH+2Cu��OH��2$\stackrel{��}{��}$CH3CH2COONa+Cu2O��+3H2O��
���� ���⿼���л�����ƶϣ���Ŀ�ѶȲ�����ע������л���������Լ��л��ﷴӦ�������ж��л�������࣬��Ҳ����������ѧϰ��ע������л���Ľṹ�������Լ���Ӧ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��

| A�� | ��NaCl�����У���Na+�����Cl-�γ��������� | |
| B�� | ��CaF2�����У�Ca2+��F-����λ���ֱ���4��8 | |
| C�� | �ڽ��ʯ�����У�̼ԭ����̼̼�������ı�Ϊ1��2 | |
| D�� | ����̬�Ŵط��ӵķ���ʽΪEF��FE |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | lmol FeI2������������Ӧʱת�Ƶĵ�����Ϊ2NA | |
| B�� | ��ϩ�ͱ�ϩ��ɵ�42 g�����������ԭ�ӵĸ���Ϊ6 NA | |
| C�� | 1 mol Na2O2�����к���������Ϊ4NA | |
| D�� | 2 L0.5 mol•L-1�������Һ�����������������ΪNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �к��ȵIJⶨ�Ǹ�����Ҫ�Ķ���ʵ�飮ȡ0.55mol/L��NaOH��Һ50mL��0.25mol/L������50mL����ͼ��ʾ��װ���н����к��ȵIJⶨʵ�飬�ش��������⣺
�к��ȵIJⶨ�Ǹ�����Ҫ�Ķ���ʵ�飮ȡ0.55mol/L��NaOH��Һ50mL��0.25mol/L������50mL����ͼ��ʾ��װ���н����к��ȵIJⶨʵ�飬�ش��������⣺| �¶� ʵ������� | ��ʼ�¶�t1�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ ��t2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 29.5 | 3.4 |
| 2 | 27.0 | 27.4 | 27.2 | 32.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.2 | |
| 4 | 26.4 | 26.2 | 26.3 | 29.8 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
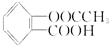 ����������NaOH��Һ��Ӧ���������NaOH�����ʵ���Ϊ��������
����������NaOH��Һ��Ӧ���������NaOH�����ʵ���Ϊ��������| A�� | 1.5mol | B�� | 2 mol | C�� | 1 mol | D�� | 0.5 mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���ۼ� | H-H | Cl-Cl | Br-Br | I-I | N��N | H-Cl | H-I | H-O | H-N |
| ���� | 436 | 243 | 194 | 153 | 946 | 432 | 299 | 463 | 391 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| �Ҵ� | 1��2-�������� | ���� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g•cm-3 | 0.79 | 2.2 | 0.71 |
| �е�/�� | 78.5 | 132 | 34.6 |
| �۵�/�� | -130 | 9 | -116 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com