
����Ŀ���ѱ���Ϊ��������֮��ĵ�����������ش��������⣺
(1)���ʯ(TiO2)���ѵ���Ҫ����֮һ����̬Tiԭ�Ӽ۲���ӵ��Ų�ͼΪ_________����̬Oԭ�ӵ���ռ������ܼ��ĵ���������ͼΪ __________�Ρ�
(2)��TiO2Ϊԭ�Ͽ��Ƶ�TiCl4��TiCl4���ۡ��е�ֱ�Ϊ205K��409K�������ڽṹ�������Ƶ�CCl4����Ҫԭ���� __________________��
(3)TiCl4������Ũ�����H2[TiCl6]������Һ�м���NH4ClŨ��Һ��������ɫ��(NH4)2[TiCl6]���塣�þ�����������֮����������� ________��
A�����Ӽ� B�����ۼ� C�����Ӽ������� D����� E�����»���
(4)TiCl4����CH3CH2OH��HCHO��CH3OCH3���л�С�����γɼӺ����������С������Cԭ�ӵ�VSEPRģ�Ͳ�ͬ���������ӵ��� _____���÷�����C�Ĺ���ӻ�����Ϊ________ ��
(5)TiO2��BaCO3һ�����ڿ��Ƶ����ᱵ��
��BaCO3�������ӵ����幹��Ϊ ________��
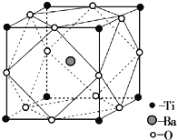
�ھ�X���߷������������ᱵ�ľ����ṹ����ͼ��ʾ��Ti4+��Ba2+����O2����Ӵ����������ᱵ�Ļ�ѧʽΪ _________����֪�����߳�Ϊa pm��O2���İ뾶Ϊb pm����Ti4+��Ba2+�İ뾶�ֱ�Ϊ____________pm��___________pm��
���𰸡�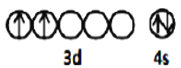 ������ TiCl4����Է�����������CCl4�����Ӽ����������� AB HCHO sp2 ƽ�������� BaTiO3
������ TiCl4����Է�����������CCl4�����Ӽ����������� AB HCHO sp2 ƽ�������� BaTiO3 ![]()
![]()
��������
(1)����Ԫ�غ�������Ų�������д�����Ų�ʽ�����ݵ����Ų�ʽ�ж�����ܼ���������������
(2)���Ӿ�����۷е�����Ӽ��������йأ�������Է������������жϣ�
(3)��Ͼ���ת����Ӧ���̣�����������жϷ�����ѧ�������ࣻ
(4)�����л�����̼ԭ�ӵijɼ���ʽ���жϿռ乹�ͣ������ж�̼ԭ���ӻ���ʽ��
(5)��Ӧ���ӻ�������ۼ�������ԭ�ӵļ۵��Ӷ���ȷ���ӻ���ʽ����ȷ�����幹�ͣ�
�ڽ�Ͼ���ͼʾ���㾧���и�ԭ�ӵĸ�����д�����ʽ���ٽ�Ͼ��������λ�ù�ϵ�������İ뾶��
(1) TiΪ38��Ԫ�أ���̬��ԭ�Ӻ�������Ų�ʽΪ��[Ar]3d24s2����۲�����Ų�ͼΪ![]() ����̬Oԭ�Ӻ�������Ų�ʽΪ1s22s22p4������ܼ�Ϊp�������������Ϊ�����ͣ�
����̬Oԭ�Ӻ�������Ų�ʽΪ1s22s22p4������ܼ�Ϊp�������������Ϊ�����ͣ�
(2) TiCl4���ۡ��е�ֱ�Ϊ205K��409K����CCl4�ṹ���ƣ������ڷ��Ӿ��壬���Ӿ�����۷е�����Ӽ��������йأ���Է�������Խ���Ӽ�������Խ���۷е�Խ�ߣ�TiCl4����Է�����������CCl4�����Ӽ�����������
(3)����ת������TiCl4������Ũ�����H2[TiCl6]���ɿ����γ�һ���ᣬ���е��ᶼ���ڹ��ۻ��������Һ�м���NH4ClŨ��Һ��������ɫ��(NH4)2[TiCl6]���壬�ɿ�������η�Ӧ����(NH4)2[TiCl6]�������к���笠����ӣ��������Ϸ�����(NH4)2[TiCl6]�����к��й��ۼ������Ӽ����ʴ�ѡAB��
(4) CH3CH2OH��CH3OCH3�е�̼ԭ�Ӷ����Ե�����ʽ�ɼ����ṹ��������ƣ�����������ṹ��HCHO��̼ԭ�Ӻ���̼��˫����������������ͬһƽ�棬Ϊƽ�������Σ����ݹ��Ϳ�֪������������Cԭ�ӵ�VSEPRģ�Ͳ�ͬ���������ӵ���HCHO�����ݹ��Ϳɵã��÷�����C�Ĺ���ӻ�����Ϊsp2�ӻ���
(5)��BaCO3��������ΪCO32-������ԭ��Ϊ̼ԭ�ӣ���۲���Ӷ���=3+![]() =3��̼ԭ��Ϊsp2�ӻ�������������4��ԭ�ӹ��ɣ������幹��Ϊƽ�������Σ�
=3��̼ԭ��Ϊsp2�ӻ�������������4��ԭ�ӹ��ɣ������幹��Ϊƽ�������Σ�
�ڸ��ݾ���ͼʾ��Tiλ�ھ����Ķ��㣬Ti����Ŀ=8��![]() =1��Baԭ��λ�ھ������ڲ�����ĿΪ1�������������ʽ�����Ӱ뾶��Oԭ��λ�ھ��������ϣ�����Ŀ=12��
=1��Baԭ��λ�ھ������ڲ�����ĿΪ1�������������ʽ�����Ӱ뾶��Oԭ��λ�ھ��������ϣ�����Ŀ=12��![]() =3���������ᱵ�Ļ�ѧʽΪBaTiO3����֪�����߳�Ϊa pm��O2���İ뾶Ϊbpm������ͼʾ�������߳�= 2r(Ti4+)+2r(O2-)=apm����r(Ti4+)=
=3���������ᱵ�Ļ�ѧʽΪBaTiO3����֪�����߳�Ϊa pm��O2���İ뾶Ϊbpm������ͼʾ�������߳�= 2r(Ti4+)+2r(O2-)=apm����r(Ti4+)=![]() pm��������Խ��ߵij���=2r(O2-)+2r(Ba2+)=
pm��������Խ��ߵij���=2r(O2-)+2r(Ba2+)=![]() a pm��r(Ba2+)=
a pm��r(Ba2+)=![]() pm��
pm��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и����Ȼ�ѧ����ʽ�У���ѧ��Ӧ����H ǰ��С�ں��ߵ���
��C��s��+O2��g���TCO2��g����H1 C��s��+![]() O2�TCO��g����H2
O2�TCO��g����H2
��S��g��+O2��g���TSO2��g����H3 S��s��+O2��g���TSO2��g����H4
��2H2��g��+O2��g���T2H2O��l����H5 H2��g��+![]() O2��g���TH2O��l����H6
O2��g���TH2O��l����H6
��CaCO3��s���TCaO��s��+CO2��g����H7 CaO��s��+H2O��l���TCa��OH��2��s����H8
A.�٢�B.��C.�ڢۢ�D.�٢ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����߶ȸ��ӻ�ѧϵͳģ�Ϳ��������ӻ�ѧ����С������(������̵�ʱ�̵�ø��)�Ļ�ѧ��Ӧ��
(1)�̵�ø�������������������֣����Dz����ܹ���N2��ԭ��NH3�����ܽ�����������Ȳ����ԭ����ϩ��
����Ȳ��__________(�����Ǽ���������������)���ӡ�
��̼������CH3-�����幹��Ϊ____________��
�����ݵȵ���ԭ����NO���ĵ���ʽΪ________________��
(2)�������ںϳɵ�ص缫��Ҳ�������˹��ϳɶ��۵ķ��̵�ø(�ṹ��ͼa)��
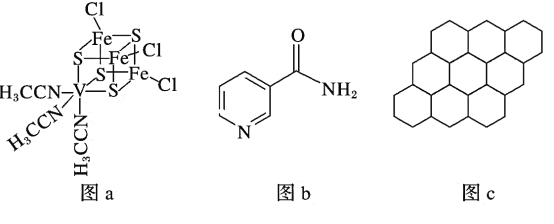
��V2����̬ʱ��������Ų�ʽΪ____________________________________________��
�����̵�ø�з�����λԭ����_____________________________(��Ԫ�ط���)��
(3)������(�ṹ��ͼb)�����ںϳɹ�ϸ�øNADPH�������������е�ԭ�ӵ��ӻ����������_______________________��1 mol�÷����к���������ĿΪ________��
(4)12 gʯīϩ(�ṹ��ͼc)�к��е�����������ĿԼΪ________������Ԥ����Ƿ������γ�����ʯīϩ�Ľṹ����˵�����ɣ�___________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼΪ�ҹ�Բ������ͭ���������ף�����ȥ���������������ж���ԭ��ķ�������ܵ��ǣ� ��

A.���DZ����ͭ������������ҺϴȥB.���õ��ԭ������������һ����ʴ�Ļƽ�
C.ͭ�Ļ�Ժ�����������ɷֲ���ӦD.�����Ǻ�һ����������������п��ͭ�Ͻ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������д���¯��ת����Ӧ��2SO2��g��+O2��g��![]() 2SO3��g�����о����֣�SO3������������¶���T���ı仯������I��ʾ�������ж���ȷ����
2SO3��g�����о����֣�SO3������������¶���T���ı仯������I��ʾ�������ж���ȷ����
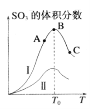
A���÷�Ӧ������ӦΪ���ȷ�Ӧ
B����Ӧ�ﵽB��ʱ��2������O2��=������SO3��
C������I��A��C���㷴Ӧ���ʵĹ�ϵ����A>��C
D����֪V2O5�Ĵ�Ч����Fe2O3�ã���I��ʾ��V2O5������ʱ�����ߣ���II��Fe2O3������ʱ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����500 mL KNO3��Cu(NO3)2�Ļ����Һ�У�c(NO3-)��6 mol/L����ʯī�缫������Һ����ͨ��һ��ʱ����������ռ���22.4 L����(��״��)���ٶ�������Һ�����Ϊ500 mL������˵����ȷ����
A. ���õ���Cu�����ʵ���Ϊ0.5 mol
B. ��������Һ�м���98 g��Cu(OH)2�ɻָ�Ϊԭ��Һ
C. ԭ�����Һ��c(K��)��4 mol/L
D. ������Һ��c(H��)��2 mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��
��1���������������г������ʣ�
��ʳ�� ��ʳ���� ���iù�� �ܼ��� �������� ������
��Ҫ�������������գ�
����ζ������___________���������ص���___________�����������ʵ���___________������ά���ص���___________��������֬����___________�����ںϳɲ��ϵ���___________��
��2��һ������50 kg�Ľ����ˣ�����Լ����2 g������2 g���������ڲ����Ե��ʵ���ʽ���ڣ�������Fe2+��Fe3+����ʽ���ڡ��������������ױ����գ���ƶѪ�߲�����ʱ��Ӧ���躬Fe2+�������Σ�����������������ά����C����ʹʳ���е�Fe3+��ԭ��Fe2+�� �������������ա�
i.�������н���Fe2+ ![]() Fe3+��ת��ʱ�����е�Fe2+��________ (�����������ԭ����)�����е�Fe3+��________ (�����������ԭ����)��
Fe3+��ת��ʱ�����е�Fe2+��________ (�����������ԭ����)�����е�Fe3+��________ (�����������ԭ����)��
ii.����ά����C����ʹʳ���е�Fe3+��ԭ��Fe2+��仰ָ��,ά����C����һ��Ӧ���� ____________�������������ԭ������
iii.�г����۵�ij����Ƭ�к������Ŀ���ϸС�Ļ�ԭ���ۣ���Щ����������θ�� (HC1)��������ת���������Ρ��˷�Ӧ�����ӷ���ʽΪ___________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����콢����Ҫ���������Ͳ��ϣ������ļװ�ҲҪ���������Ҫ��ʴ�����������Ͻ���Ǿ��и�ǿ�ȡ����¡���ʴ���������ܵ����ָ֣��������ָ��к���̼���衢����������Ԫ����
��1�������ֿ���ʴ����ǿ��![]() ��̬ԭ�ӵļ۵����Ų�_________��Ϊ��Ԫ�������ڱ���_________����
��̬ԭ�ӵļ۵����Ų�_________��Ϊ��Ԫ�������ڱ���_________����
��2��![]() ���γɶ��������ӣ���
���γɶ��������ӣ���![]() ��
��![]() ��
��![]() �ȣ�
�ȣ�![]() ����ԭ�ӵ���λ����_________����
����ԭ�ӵ���λ����_________����![]() ��Ϊ�ȵ�����ķ���Ϊ_________��
��Ϊ�ȵ�����ķ���Ϊ_________��
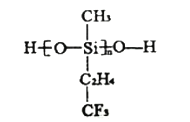
��3�������װ�Ϳ��һ�����µIJ��Ͼ۹�����ṹ��ͼ��ʾ������![]() ԭ���ӻ���ʽΪ________�ӻ���
ԭ���ӻ���ʽΪ________�ӻ���
��4��̼��ɻ��ϳ�̼���裬![]() ����������ƽ��ʯ�Ľṹ������̼ԭ�Ӻ�ԭ�ӵ�λ���ǽ���ģ�����̼������۵���ڽ��ʯ��ԭ����_________��
����������ƽ��ʯ�Ľṹ������̼ԭ�Ӻ�ԭ�ӵ�λ���ǽ���ģ�����̼������۵���ڽ��ʯ��ԭ����_________��
��5������������֮һ��![]() ����
����![]() ��ȡ�����������侧��ṹ��һ�������壬�����е������Ƿ��������������ܶѻ���________����ǡ��������������Dz���
��ȡ�����������侧��ṹ��һ�������壬�����е������Ƿ��������������ܶѻ���________����ǡ��������������Dz���![]() �ľ�����_________����ǡ������������������Ӵ���������Χ�ɵ�_________����ռ�ṹ����϶��������ͼ����
�ľ�����_________����ǡ������������������Ӵ���������Χ�ɵ�_________����ռ�ṹ����϶��������ͼ����![]() ������ܶ�Ϊ_________
������ܶ�Ϊ_________![]() ����ͼ
����ͼ![]() ��������������λ��Ч���֣�
��������������λ��Ч���֣�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�����ڷǽ���Ԫ�ص�ԭ�Ӻ��������������Ǵ�����������һ�룬��Ԫ��
A. ����Ȼ����ֻ�Ի���̬����ʽ����
B. ���ʳ�������뵼����Ϻ��ά
C. ��������ﲻ���ᷴӦ
D. ��̬�⻯��ȼ����ȶ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com