
����Ŀ���й���ѧ���ںϳɰ���N2+3H2![]() 2NH3��H<0����Ӧ�����о���ȡ���½�չ���״α�����LiH-3d���ɽ�����һ���ϴ�����ϵ�������������ת��������������ͼ��ʾ������˵������ȷ����
2NH3��H<0����Ӧ�����о���ȡ���½�չ���״α�����LiH-3d���ɽ�����һ���ϴ�����ϵ�������������ת��������������ͼ��ʾ������˵������ȷ����
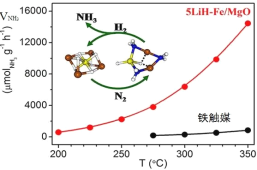
A.ת���������зǼ��Լ��������γ�
B.���ϴ��������˷�Ӧ�Ļ��
C.���ϴ����ܽ��ͺϳɰ���Ӧ���ʱ�
D.�����ºϳɰ��������ԭ��ת����
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij������ӵ�ص����������ǽ���������ﮣ�LiCoO2���������۾���Ϳ�����������Ƴɵģ������������ã�ijУ�о�С�鳢�Ի��շϾ����������е��ܡ�
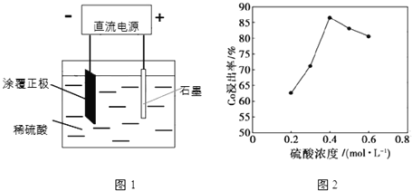
(1)25��ʱ����ͼ1��ʾװ�ý��е�⣬��һ����������Co2+����ʽ���������н������������������ݲ�����һ��ʱ������������������롣
�������ĵ缫��ӦʽΪ��LiCoO2+4H++e-=Li++Co2++2H2O�������ĵ缫��ӦʽΪ______��
�ڸ��о�С�鷢������Ũ�ȶ��ܵĽ������нϴ�Ӱ�죬һ�������£������仯������ͼ2��ʾ����c��H2SO4����0.4molL-1ʱ���ܵĽ������½�����ԭ�����Ϊ______��
(2)�����ɺ�õ���Co2+�Ľ���Һ���������������۳����ڵ��۵ײ��������²�����������ܡ�

��д��������������������۷�����Ӧ�Ļ�ѧ����ʽ______���ò���һ����80�����½��У��¶Ȳ���̫�ߵ�ԭ����______��
����֪��NH4��2C2O4��Һ�������ԣ����й�ϵ����ȷ����______������ĸ��ţ���
a��c(NH4+)��c(C2O42-)��c(H+)��c(OH-)
b��c(H+)+c(NH4+)=c(OH-)+c(HC2O4-)+c(HC2O42-)
c��c(NH4+)+c(NH3H2O)=2[c(HC2O42-)+c(HC2O4-)+c(H2C2O4)]
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����0.1000 mol��L1 NaOH��Һ�ζ�20.00 mL 0.1000 mol��L1��H3A��Һ�ĵζ�������ͼ��ʾ����֪H3A��pKa1��pKa2��pKa3�ֱ�Ϊ2��7��12(pKa����lgKa)������˵����ȷ����
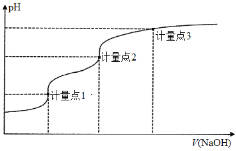
A.�ζ�ǰ��ҺpHֵԼΪ3
B.������1ʱ�������ü�����Ϊָʾ��
C.���������2ʱ����Һ�д���c(Na��)��c(H2A��)��2c(HA2��)��3c(A3��)
D.���������3ʱ������Һ��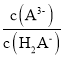 ������
������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������ȷ���ǣ� ��
A.�����£���pH=4������1mLϡ����100 mL��pH����6
B.�����£�pH��Ϊ3�Ĵ����������Һ�������Ũ�ȴ��������Ũ��
C.�����£�pHΪ1��������Һ�м�������pHΪ13����������Һǡ����ȫ�к�
D.��V1LpH=11��NaOH��Һ��V2LpH=3��HA��Һ��Ϻ������ԣ���V1��V2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ζ�ʵ���ǻ�ѧѧ������Ҫ�Ķ���ʵ�飬������ԭ�ζ�ʵ��������к͵ζ����ơ���Ѫ�Ƶĺ���ʱ����������ʵ�飺
�ٿɽ�4mLѪҺ������ˮϡ�ͺ������м������������(NH4)2C2O4���壬��Ӧ����CaC2O4��������������ϡ���ᴦ����H2C2O4��Һ��
�ڽ��ٵõ���H2C2O4��Һ����������KMnO4��Һ�ζ�����������ΪCO2����ԭ����ΪMn2+��
���յ�ʱ��ȥ20mL l.0��l0-4mol/L��KMnO4��Һ��
(1)д��KMnO4��Һ�ζ�H2C2O4��Һ�����ӷ���ʽ______________________________________���ζ�ʱ��KMnO4��ҺӦװ��______������������������ʽ�ζ����У��ζ��յ�ʱ�ζ�������_________________________��
(2)���в����ᵼ�²ⶨ���ƫ�͵���_________��
A���ζ�����װҺǰδ�ñ�KMnO4��Һ��ϴ
B���ζ������У���ƿҡ����̫���ң���ƿ����Һ�ν���
C���ζ��ܼ��첿���ڵζ�ǰû�����ݣ��ζ��յ�ʱ��������
D���ﵽ�ζ��յ�ʱ�����Ӷ���
(3)���㣺ѪҺ�к������ӵ�Ũ��Ϊ_________mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵���������(��)
A. ���е�ԭ�Ӷ��������ӡ����Ӻ͵������ֻ���������
B. �����ը�������⡢�����Ԫ��
C. ![]() ��ͼ��ԭ��ģ���Dz��������
��ͼ��ԭ��ģ���Dz��������
D.  ģ���е�С�ڵ��ʾ������ԭ�Ӻ�����ֵĸ����ܶȵ���������
ģ���е�С�ڵ��ʾ������ԭ�Ӻ�����ֵĸ����ܶȵ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��
ͭ�����ͽ����ճ������г��ý���
��1����̬ͭԭ�ӵļ۲�����Ų�ʽΪ___________.
��2��������Һ��Ҫ�ɷ���[Ag��NH3��2]OH�����Ʒ����ǣ���AgNO3��Һ�еμӰ�ˮ�������պ���ȫ�ܽ�Ϊֹ���õ������������Һ
��AgNO3�������ӵĿռ乹����_______________��
��[Ag��NH3��2]+�������ӵ���λ��Ϊ___________��NH3������N���ӻ�������__________��
����NH3��Ϊ�ȵ�����������У�_____________��
��3���ִ���ҵұ���У�2Au��CN��2��+Zn====2Au+Zn��CN��42����CN���dz��������壬�ṩ�µ��Ӷ���C����N������Ҫԭ����_________________________________��
��4��ͭ�������л���Ӧ�����Ĵ����� CH3CH2OH ![]() CH3CHO+H2����CH3CH2OH�ķе����CH3CHO����Ҫԭ����________����ԭ�ӹ���ص���ʽ���࣬H2����������������____________��
CH3CHO+H2����CH3CH2OH�ķе����CH3CHO����Ҫԭ����________����ԭ�ӹ���ص���ʽ���࣬H2����������������____________��
��5��һ��ͭ���Ͻ��׳ư�ͭ���ľ�����ͼ1��ʾ��ͭ����ԭ�Ӹ�����Ϊ___________��

��6������ͼ2��ʾ�����־���ѻ���ʽ��Ϊ___________�ѻ����þ�����ԭ�ӿռ������ʣ�![]() ��Ϊ___________ ����������ʽ�ӱ�ʾ��������ʾԭ�ӿռ�������=
��Ϊ___________ ����������ʽ�ӱ�ʾ��������ʾԭ�ӿռ�������=![]() ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2019���ǡ����ʻ�ѧԪ�����ڱ��ꡱ��1869���Ž��з�ѵ�ʱ��֪��Ԫ�ظ�����������ѧ���ʽ������У�ȷԤ���˼ס�������δ֪Ԫ�ص�λ�ã���Ԥ���˶��ߵ����ԭ������������ԭʼ��¼���£�

����˵������ȷ����
A.Ԫ�ؼ�λ������Ԫ�����ڱ��������ڵڢ�A��
B.Ԫ���ҵļ���̬�⻯����ȶ���ǿ��CH4
C.ԭ�Ӱ뾶�Ƚϣ��ף��ң�Si
D.�Ʋ��ҿ��������뵼�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������M���Ʊ�һ�ֿ���ҩ���м��壬ʵ�����Է��㻯����AΪԭ���Ʊ�M��һ�ֺϳ�·�����£�
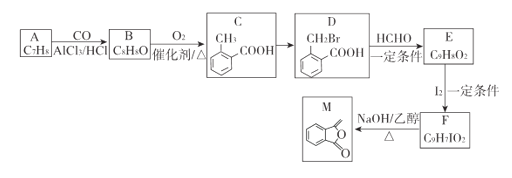
��֪��R1CH2Br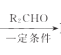 R1CH=CHR2
R1CH=CHR2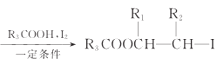
�ش��������⣺
(1)A�Ľṹ��ʽΪ_______��B�й����ŵ�����Ϊ_______��C�Ļ�ѧ����Ϊ_________��
(2)��C����D������Լ�������Ϊ_______���÷�Ӧ����Ϊ____________.
(3)��F����M�Ļ�ѧ����ʽΪ__________________________________��
(4)QΪM��ͬ���칹�壬��������������Q�Ľṹ��________��(���������칹)����д������һ.�ֺ˴Ź�����������4�����շ�Ľṹ��ʽ_________________��
�ٳ���������������״�ṹ
���ܷ���ˮ�ⷴӦ��������Ӧ
(5)���������ϳ�·�ߺ���Ϣ������ϩ����ȩΪԭ��(���Լ���ѡ)������Ʊ���2-��ϩ�ĺϳ�·��_____________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com