
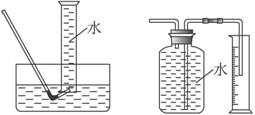
������������⣺
��1����μ��װ��A�������ԣ�___________________________________________________��
��2���μ�����ʱ�����ַ�Ӧ���ʽ�֮ͬŨ�������봿���۷�ӦҪ�죬��ԭ����____________________________________________________________________��
��3�����ձ�����Һ��������Ũ�����ٽ��½ᾧ���Ƶ�FeCl3��6H2O���壬������ֱ�������ᾧ�ķ������Ƶþ����������___________________________________________________��
��4���ø÷��Ƶõľ�������������Fe��NO3��3��Ϊ���Ƶýϴ�����FeCl3��6H2O���ɽ��ձ��ڵ�ϡ���ỻ��_____________________________________________________________��
��5����Ҫ�����м�Ĵ��ȣ��ɲ����B�зų���������V��������ɱ������λ��L���������м�Ĵ���Ϊ��____________����m��V�Ĵ���ʽ��ʾ�������ڿ�ͼC���л�����Ҫ��װ�á�
��1���ٹرջ���a������b����Һ©���������϶����ӣ�����עˮ��©���¶˻��γ�һ��Һ����һ��ʱ�����Һ���������ƣ�˵�������Բ��ã���Һ��ͣס��������˵�����������á��ڹرշ�Һ©�������ͻ���b������a�����ܿ�B��һ�δ������ܵ��齺�ܲ�����ˮ��ˮ�У�˫����סװ��A����ˮ���������ݲ������ſ�˫�ֲ��������γ�һ��Һ����������������
��2������������ͭ�γ�ԭ��ط�Ӧ���ӿ��˷�Ӧ����
��3����ΪFeCl3��ǿ�����������Իᷢ�����µ�ˮ�ⷴӦ��FeCl3+3H2O![]() Fe��OH��3+3HCl����������ʱʹHCl�ӷ����ˮ��ƽ�����ƣ���˵ò���FeCl3��6H2O
Fe��OH��3+3HCl����������ʱʹHCl�ӷ����ˮ��ƽ�����ƣ���˵ò���FeCl3��6H2O
��4��˫��ˮ��������ˮ
��5��![]() ��
��![]()




| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��д���пհף�
(1)��μ��װ��A�������ԣ�________________________��
(2)�μ�����ʱ�����ַ�Ӧ���ʽ�֮ͬŨ�������봿���۷�ӦҪ�죬��ԭ����_____________
___________________��
(3)���ձ�����Һ��������Ũ�����ٽ��½ᾧ���Ƶ�FeCl3��6H2O���壬������ֱ�������ᾧ�ķ������Ƶþ����������_______________________________________________________��
(4)�ø÷��Ƶõľ�������������Fe (NO3)3��Ϊ���Ƶýϴ�����FeCl3��6H2O���ɽ��ձ��ڵ�ϡ���ỻ��________________________________________��
(5)��Ҫ�����м�Ĵ��ȣ��ɲ����B�зų���������V (������ɱ�״������λ��L)�������м�Ĵ���Ϊ__________(�ú�m��V�Ĵ���ʽ��ʾ)�����ڿ�ͼC�л�����Ҫ��װ�á�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���Ȼ�������ѧ��ѧʵ�����бز����ٵ���Ҫ�Լ���ijͬѧ���÷���м��������ͭ�Ȳ������ᷴӦ�����ʣ����Ʊ�FeCl3��6H2O����ͬѧ��Ƶ�ʵ��װ����ͼ��ʾ��A�з���m�˷���м���ձ���ʢ�й�����ϡ���ᣬʵ��ʱ��a���ر�b���ӷ�Һ©������A�мӹ�����ϡ���ᣬ��ʱ��Һ��dz��ɫ���ٴ�b���й��ˣ����˽�����ȡ�ձ�����Һ������������ȣ�����������ˮ��ʹ����HNO3�ֽ⣬�ٽ��½ᾧ��FeCl3��6H2O���塣
��д���пհף�
��1����μ��װ��A�������ԣ� ��
��2���μ�����ʱ�����ַ�Ӧ���ʽ�֮ͬŨ�����������۷�ӦҪ�죬��ԭ���� ��
��3�����ձ�����Һ��������Ũ�����ٽ��½ᾧ���Ƶ�FeCl3��6H2O���壬������ֱ�������ᾧ�ķ������Ƶþ�������� ��
��4���ø÷��Ƶõľ�������������Fe��NO3��3��Ϊ���Ƶýϴ�����FeCl3��6H2O���ɽ��ձ��ڵ�ϡ���ỻ�� ��
��5����Ҫ�����м�Ĵ��ȣ��ɲ����B���ų����������������������ʱ�����������ȴ������ʱ���У���������װ�òⶨ��B���ų��������������Ե�������Ͳ����ռ���������Ӧѡ�� ������ţ��������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ������ѧ������ѧ�ڽμ�⻯ѧ�Ծ� ���ͣ������
���Ȼ�������ѧ��ѧʵ�����бز����ٵ���Ҫ�Լ���ijͬѧ���÷���м��������ͭ�Ȳ������ᷴӦ�����ʣ����Ʊ�FeCl3��6H2O����ͬѧ��Ƶ�ʵ��װ����ͼ��ʾ��A�з���m�˷���м���ձ���ʢ�й�����ϡ���ᣬʵ��ʱ��a���ر�b���ӷ�Һ©������A�мӹ�����ϡ���ᣬ��ʱ��Һ��dz��ɫ���ٴ�b���й��ˣ����˽�����ȡ�ձ�����Һ������������ȣ�����������ˮ��ʹ����HNO3�ֽ⣬�ٽ��½ᾧ��FeCl3��6H2O���塣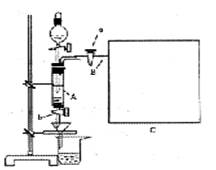
��д���пհף�
��1����μ��װ��A�������ԣ� ��
��2���μ�����ʱ�����ַ�Ӧ���ʽ�֮ͬŨ�����������۷�ӦҪ�죬��ԭ���� ��
��3�����ձ�����Һ��������Ũ�����ٽ��½ᾧ���Ƶ�FeCl3��6H2O���壬������ֱ�������ᾧ�ķ������Ƶþ�������� ��
��4���ø÷��Ƶõľ�������������Fe��NO3��3��Ϊ���Ƶýϴ�����FeCl3��6H2O���ɽ��ձ��ڵ�ϡ���ỻ�� ��
��5����Ҫ�����м�Ĵ��ȣ��ɲ����B���ų����������������������ʱ�����������ȴ������ʱ���У���������װ�òⶨ��B���ų��������������Ե�������Ͳ����ռ���������Ӧѡ�� ������ţ��������� ��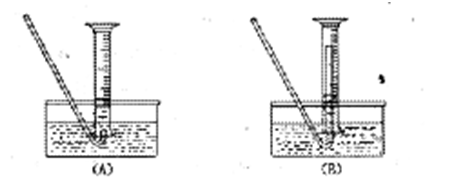
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ������ѧ�ڽμ�⻯ѧ�Ծ� ���ͣ������
���Ȼ�������ѧ��ѧʵ�����бز����ٵ���Ҫ�Լ���ijͬѧ���÷���м��������ͭ�Ȳ������ᷴӦ�����ʣ����Ʊ�FeCl3��6H2O����ͬѧ��Ƶ�ʵ��װ����ͼ��ʾ��A�з���m�˷���м���ձ���ʢ�й�����ϡ���ᣬʵ��ʱ��a���ر�b���ӷ�Һ©������A�мӹ�����ϡ���ᣬ��ʱ��Һ��dz��ɫ���ٴ�b���й��ˣ����˽�����ȡ�ձ�����Һ������������ȣ�����������ˮ��ʹ����HNO3�ֽ⣬�ٽ��½ᾧ��FeCl3��6H2O���塣
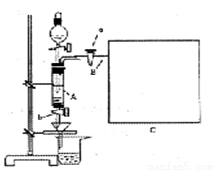
��д���пհף�
��1����μ��װ��A�������ԣ� ��
��2���μ�����ʱ�����ַ�Ӧ���ʽ�֮ͬŨ�����������۷�ӦҪ�죬��ԭ���� ��
��3�����ձ�����Һ��������Ũ�����ٽ��½ᾧ���Ƶ�FeCl3��6H2O���壬������ֱ�������ᾧ�ķ������Ƶþ�������� ��
��4���ø÷��Ƶõľ�������������Fe��NO3��3��Ϊ���Ƶýϴ�����FeCl3��6H2O���ɽ��ձ��ڵ�ϡ���ỻ�� ��
��5����Ҫ�����м�Ĵ��ȣ��ɲ����B���ų����������������������ʱ�����������ȴ������ʱ���У���������װ�òⶨ��B���ų��������������Ե�������Ͳ����ռ���������Ӧѡ�� ������ţ��������� ��

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com