
����Ŀ��Se���ƹ��ص�һ��ԭ�ϡ����ͭ���������к���3%-14%��SeԪ��(SeԪ����Se���ʺ�Cu2Se����ʽ����)��������ϡ�н������������������������ȡSe���������£�
![]()
��ش��������⣺
(1)Ũ�����ܽ�Cu2Se����CuSO4��SO2��SeO2�Ļ�ѧ����ʽΪ__________________��
(2)���̢ڵ�Ŀ����_____________________��
(3)���̢ܵIJ���������_____________________��
(4)SO2��SeO2(g)��Ӧ�����ӷ���ʽΪ________________������������ȡSe�Ĺ������̿�֪��SeO2��H2SO4(Ũ)��SO2����������ǿ������˳����_____________��
(5)��ҵ��ȡSe�������У���ѭ�����õ�������________________��(�ѧʽ)
(6)�ٳ�ȡ5.000g���ͭ��������Ʒ���Ժ��ʷ����ܽ⣬���250.0mL������Һ
����ȡ������Һ25.00mL����ƿ�У�����30.0mL0.0100mol/LKMnO4����Һ(Se��+4��ת��Ϊ+6��)��
�۷�Ӧ��ȫ����0.05000mol/L(NH4)2Fe(SO4)2����Һ�����յ㣬����(NH4)2Fe(SO4)2����Һ10.00mL��
����ͭ��������Ʒ��Se����������Ϊ__________________������FeCl2����Һ����(NH4)2Fe(SO4)2����Һ���еζ����ԲⶨSe�������������__________(�䡰ƫ�ߡ���ƫ�͡��� ��Ӱ�족)
���𰸡� Cu2Se+6H2SO4(Ũ) ![]() 2CuSO4+SeO2+4SO2��+6H2O�� ʹSO2��SeO2�ӷ����� ���� SeO2(g)+2SO2+2H2O=Se��+2SO42-+4H+ H2SO4(Ũ)>SeO2>SO2 H2SO4 7.9% ƫ��
2CuSO4+SeO2+4SO2��+6H2O�� ʹSO2��SeO2�ӷ����� ���� SeO2(g)+2SO2+2H2O=Se��+2SO42-+4H+ H2SO4(Ũ)>SeO2>SO2 H2SO4 7.9% ƫ��
��������������Ҫ������ڴ�����������ȡSe�����̵����ۡ�
(1)Ũ�����ܽ�Cu2Se����CuSO4��SO2��SeO2�Ļ�ѧ����ʽΪCu2Se+6H2SO4(Ũ) ![]() 2CuSO4+SeO2+4SO2��+6H2O����
2CuSO4+SeO2+4SO2��+6H2O����
(2)���̢ڵ�Ŀ����ʹSO2��SeO2�ӷ�������
(3)���̢ܵIJ��������ǹ�����
(4)��ͼ��֪SO2��SeO2(g)��Ӧ����Se��H2SO4����Ӧ�����ӷ���ʽΪSeO2(g)+2SO2+2H2O=Se��+2SO42-+4H+������������ȡSe�Ĺ������̿�֪��SeO2��H2SO4(Ũ)��SO2����������ǿ������˳����H2SO4(Ũ)>SeO2>SO2��
(5)��ҵ��ȡSe�������У���ѭ�����õ�������H2SO4��
(6) ��(NH4)2Fe(SO4)2����Һ��Ӧ����KMnO40.05000mol/L��10.00mL/5=0.1mmol���������Һ��Ӧ����KMnO430.0mL��0.0100mol/L-0.05000mol/L��10.00mL/5=0.2mmol����KMnO4����Һ��Ӧ����Se�����ʵ���Ϊ2.5��0.2mmo=0.5mmol������ͭ��������Ʒ��Se����������Ϊ0.5mmol��250.0mL/25.00mL��g/mol/5.000g=7.9%������FeCl2����Һ����(NH4)2Fe(SO4)2����Һ���еζ�������FeCl2�ױ���������������������KMnO4����Һ�����С����ʹSe�����������IJⶨ���ƫ����


 ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijʵ��С���о���Һ��AgNO3��Na2S�ķ�Ӧ��
ʵ�� | �Լ� | ���� | |
| �Թ� | �ι� | |
��pH = 4�� |
��pH = 9�� | ���ֺ�ɫ���� | |
��1�������ӷ���ʽ����Na2S��ҺpH > 7��ԭ��________��
��2��ʵ��С��ͬѧ��Ϊ��ɫ�����п��ܺ���Ag2O��Ag2S��Ag�����ʵ����֤��
��֪��i��Ũ�����ܽ�Ag2Sת��Ϊ![]() ��
��![]() ��
��
ii��Ag2O���ܽ���Ũ��ˮ���γ�������Һ����Ag2S��Ag�����ܡ�
�� ��Ʋ�ʵʩ����ʵ�飬֤ʵ�����к���Ag2S��

�Լ�1���Լ�2�ֱ���_________��_________��
����1������2�ֱ���_________��_________��
�� ��Ʋ�ʵʩ����ʵ�飬֤ʵ������������Ag2O����ʵ�����������������
ʵ����� | ʵ������ | |
����i | ȡ����������Һ�������еμ����� | ���ְ�ɫ���� |
����ii | ȡ����ϴ�Ӻ�ĺ�ɫ������____________ | ____________ |
�� �����飬����������Ag��

��3��ʵ��С��ͬѧ��ΪAgNO3��Һ���������ԣ���һ���������ܹ�����Na2S�����ʵ������о���ʵ��װ������ͼ��ʾ������õ�ѹΪa��![]() ����
����
��AgNO3��Һ������![]() �����ʽ����Ʋ⣺
�����ʽ����Ʋ⣺
����1�� ![]() ��AgNO3��Һ��
��AgNO3��Һ��![]() ������
������![]() ��
��
����2�� ![]() ��AgNO3��Һ��
��AgNO3��Һ��![]() ������
������![]() ��
��
������ͼװ�ü����о�����֪����ѹ��С��ӳ������������ԭ��ǿ���IJ��죻�����������뻹ԭ��ǿ������Խ��ѹԽ��
�� ��![]() ��AgNO3��Һ�滻Ϊ_______��Һ����¼��ѹΪb��
��AgNO3��Һ�滻Ϊ_______��Һ����¼��ѹΪb��![]() ����
����
�� ����ʵ��֤ʵ������![]() ��������һ������
��������һ������![]() ����֤����______��
����֤����______��
ʵ����ۣ�AgNO3��Һ��Na2S��Һ�ķ�Ӧ�����뷴Ӧ�����йء�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ�鷽���ܴﵽʵ��Ŀ�ĵ���
A. �õ�����Һ������Һ���Ƿ���ڵ⻯��
B. �÷�Һ©�����뱽��ƾ��Ļ����
C. ���Ȼ�����Һ����̼������Һ����������Һ
D. �ö����ЧӦ�����������������FeCl3��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CO��ȼ����ΪakJ/mol����COȼ�ղ���ͨ��һ��Ũ�ȵ�NaOH��Һ�У�������Һ�����ʽ�ΪNa2CO3��Ũ��Ϊ0.01mol/L��
��1��д��CO��ȫȼ�յ��Ȼ�ѧ����ʽ__________________________��
��2��Na2CO3��Һ�ʼ��ԣ���������ȥ���ۣ������ӷ���ʽ��ʾNa2CO3�ʼ��Ե�ԭ��___________________________________��
��3��д��Na2CO3��Һ�еĵ���غ��ϵ__________����������Ũ�ȷ��ű�ʾ��
��4�������ڸ�Ũ�ȵ�Na2CO3��Һ��c(CO32-)+c(HCO3-)+c(H2CO3)=____mol/L
��5�������Һ�еμ�AlCl3��Һ���������ݺͰ�ɫ�������ɣ�д��������Ӧ�����ӷ���ʽ__________________________��
��6����֪Cu(OH)2���ܶȻ�ΪKsp��2��10��20��ijCuSO4��Һ��c(Cu2��)��0.02 mol��L��1����Ҫ����Cu(OH)2������Ӧ������Һ��pH��ʹ֮����________��Ҫʹ0.2 mol��L��1 CuSO4��Һ��Cu2��������Ϊ��ȫ��ʹCu2��Ũ�Ƚ���ԭ����ǧ��֮һ������Ӧ����Һ�����NaOH��Һ��ʹ��ҺpHΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��С������ͼװ����ʵ��װ��(����ͷ�ι��еĹ���������Һ�����ȣ�Ũ�ȷֱ�Ϊ5%��10%)��ʵ��ʱ��ͬʱ��ȫ������ιܵĽ�ͷ�����۲�ʵ������
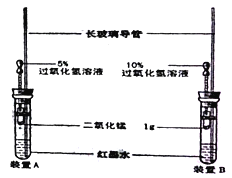
��1��С����ʵ��Ŀ���ǣ�__________________________��
��2��װ���г��������ܵ������ǣ�_________________����īˮ��������________��
��3��������������������е�ʵ��������_____________��������____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����仯�����ڲ������졢�л��ϳɵȷ�����;�dz��㷺���ش��������⣺
(1)VB2��һ�ֵ����մɲ��ϣ���̬��ԭ�ӵļ۵����Ų�ͼΪ___________
(2)B��C��N��O����Ԫ�ص�һ��������С�����˳��Ϊ_______________��
(3)���±�����ڹ�ҵ������Ҫ���ã��������±����ķе����±���ʾ��
±���� | BF3 | BCl3 | BBr3 | BI3 |
�е�/K | 172 | 285 | 364 | 483 |
������±����е��������ߵ�ԭ����_________________��
����BF3���ӽṹ���ͷ�ӦBF3(g)+NH4F(s)=NH4BF4(s)�ܹ�������ԭ��_________________��
���Ʊ�������ķ������£�
![]()
BCl3��LiBH4����ԭ�ӵ��ӻ������������Ϊ_________________����B3N3H6��Ϊ�ȵ�����ķ��ӵĽṹ��ʽΪ___________________��
(4)����������ľ����ṹ����ʯ�ṹ����(����ͼ)���dz�Ӳ���ϡ�
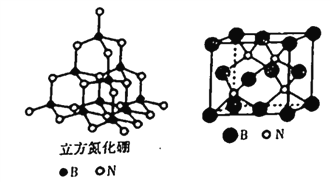
�پ�����ÿ����ԭ����Χ��������ҵȾ������ԭ����_____����
�ڽṹ��ѧ�ϳ���ԭ�����������ʾ�����ڲ���ԭ�ӵ����λ�ã�����������ľ����У� Bԭ�ӵ���������ֱ��У�B(0��0��0)��B(![]() ��0��
��0�� ![]() )��B(0��
)��B(0�� ![]() ��
�� ![]() )�ȣ��������������Bԭ������ҵȾ����Nԭ�ӵ��������Ϊ___________________��
)�ȣ��������������Bԭ������ҵȾ����Nԭ�ӵ��������Ϊ___________________��
����֪�������߳�Ϊapm����������ܶ�Ϊ____g��cm-3(��NAΪ�����ӵ�����ֵ��ֻҪ������ʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͨˮ���ڹ̻�������������ˮ���Ӽ��ٲ��γɼ�����Һ��������һ������ѧ�ص㣬��ѧ�ҷ����˵綯�Ʒ���ˮ��ij���ʱ�䡣�˷���ԭ����ͼ��ʾ����Ӧ���ܷ���ʽΪ2Cu��Ag2O===Cu2O��2Ag�������й�˵����ȷ���� (����)
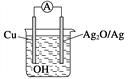
A. 2 mol Cu��1 mol Ag2O������������1 mol Cu2O��2 mol Ag���������
B. �����ĵ缫��ӦʽΪ2Cu��2OH����2e��===Cu2O��H2O
C. ����ԭ��ʾ��ͼ�У����������Cu��Ag2O
D. ��ع���ʱ��OH���������ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ѧ��ѧ����Ԫ�ص�ԭ�ӽṹ���������±���ʾ:
Ԫ�� | �ṹ������ |
A | A�����������еij������������������Ȼ����Է����������35.5 |
B | Bԭ���������������ڲ����������1/5 |
C | C�dz������ʵ���ҪԪ�أ����ʳ����³���̬ |
D | D�ǵؿ��к�����ḻ�Ľ���Ԫ�أ���ҵ�Ͽ�ͨ����ⷨ��ȡ�䵥�� |
E | ͨ������£�Eû�������ϼۣ�A��B��C��D��F������E�γɻ����� |
F | F�����ڱ��п�������IA�壬Ҳ�����������VIIA�� |
(1)AԪ�������ڱ��е�λ��Ϊ��_____����_____����
(2)B��C�γɵĻ�����Ļ�ѧʽΪ______��������____(��������������������)�����
(3)F��E�����γ�ԭ�Ӹ����ȷֱ�Ϊ2:1��1:1�����ֻ�����X��Y������X��Y��ˮ��Һ��ʵ�鷽����_________________________________________��
(4)C��E���ǽϻ��õķǽ���Ԫ�أ����ߵķǽ�����ǿ���Ƚϣ�_____��______���û�ѧ���Żش𣩣������ٳ�һ���ж϶��߷ǽ�����ǿ����֤����____________________��
(5)������ΪB��D�ĵ����õ������Ӻ����NaOH��Һ�п����γ�ԭ��أ�����Ϊ�Ƿ���ԣ������ԣ���д�������ĵ缫����ʽ(����Ϊ���пɲ�д)_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������4����Ҫ���л��
��![]() ��
��![]() ��
��![]() ��
��![]()
��ش�
��1���ܷ���������Ӧ����______������ţ���
��2������Ũ������һ����������������ըҩTNT����______������ţ���
��3����������һ�������·�Ӧ������ϳɸ߷��ӻ��������______������ţ���
��4��д������һ�������·�����ȥ��Ӧ�Ļ�ѧ����ʽ��______��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com