
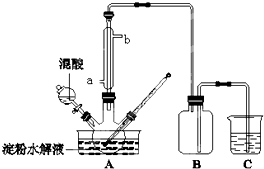
 ����ˮ��IJ��C6H12O6�����������������Ʊ����ᣬװ����ͼ��ʾ�����ȡ�����������̶�װ�þ�����ȥ����ʵ��������£�
����ˮ��IJ��C6H12O6�����������������Ʊ����ᣬװ����ͼ��ʾ�����ȡ�����������̶�װ�þ�����ȥ����ʵ��������£����� ��1��Ũ���������ˮ�Ժʹ����������������������ɲ��ᣬͨ��Ũ������ˮˮ�ִٽ�������Ӧ�����ƶ���
��2�������ܵ������������������������������ã���������ˮ������Ϊ������ʵ����������μӹ��죬������Ũ�ȹ��ᵼ��������������������ǣ�C6H12O6��������ΪCO2����H2C2O4��һ����������
��3����ˮ�������ۻ�����������Һ�����ۣ������ˮû�б仯��
��4���ú������ĸҺ�����յ�������ŵ㣺���HNO3�����ʣ���ѭ��ʹ�õ�������� ȱ�㣺NOx������������ղ���ȫ��
��5���������Ϊ�Ϻ�ɫ�������в���Ҫ��ָʾ���������������£�������������ܺͲ��ᷢ��������ԭ��Ӧ���ɶ��������ӡ�������̼��ˮ�����ݷ�Ӧ���㣻
��� �⣺��1��Ũ�������ǿ�����ԡ���ˮ�Ժ���ˮ�ԣ�����ʵ���ǽ�C6H12O6���������������Ʊ����ᣬŨ������������Ũ������ˮ�����������ɲ���ķ����ƶ�������Ũ����������Ǽӿ����ˮ������ʣ������������ã���
�ʴ�Ϊ���ӿ����ˮ������ʣ������������ã���
��2�����ܵ������������������������������ã�����Ч������Ч���ã�����ˮ�Ľ�����a��b��������Ϊ65%HNO3��98%H2SO4�Ļ��Һ�����Һ����ˮ���ȣ��¶ȸ��ܼӿ컯ѧ��Ӧ�������ܽ�һ������H2C2O4�ɶ�����̼��
�ʴ�Ϊ��a���¶ȹ��ߣ�����Ũ�ȹ�����H2C2O4��һ����������
��3����ˮ�������ۻ�����������Һ�����ۣ������ˮû�б仯�����Լ�������Ƿ�ˮ����ȫ�ķ�����ȡ����ˮ������Һ���Թ��У���1-2�ε�ˮ������Һ�з�����������˵��û��ˮ����ȫ����������˵��ˮ����ȫ��
�ʴ�Ϊ��ȡ����ˮ������Һ���Թ��У���1-2�ε�ˮ������Һ�з�����������˵��û��ˮ����ȫ����������˵��ˮ����ȫ��
��4���ú������ĸҺ�����յ������������������ظ�ʹ�ã����HNO3�����ʣ���Ҳ��������ղ���֣���ɻ�����Ⱦ��
�ʴ�Ϊ�����HNO3�����ʣ�NOx������������ղ���ȫ��
��5�����������ҺΪ�Ϻ�ɫ�����ﵽ�ζ��յ�ʱ���ٵ�����������Һʱ������ɫ������ȥ����Ϊ�Ϻ�ɫ������ɫ���������ƣ�Na2C2O4������ϡ�����У�Ȼ�������Ը��������Һ���еζ������ӷ���ʽΪ2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O��ͼʾ�ζ���������Һ���=18.50mL-2.50mL=16.00mL��
n��KMnO4��=0.016L��0.0200mol•L-1=3.2��10-4mol�����ݷ���ʽ�ɵã�
2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O
2 5
3.2��10-4mol 8��10-4mol
��Ʒ�ж�ˮ�ϲ��������Ϊm=8��10-4mol��126g/mol=8��126��10-4g=0.1008g��
��ò��ᾧ����Ʒ�ж�ˮ�ϲ������������Ϊ$\frac{0.1008g}{0.12g}$��100%=84.0%��
�ʴ�Ϊ����ɫ���Ϻ�ɫ������ɫ����84.0%��
���� ���⿼���˲������ȡ��Ϊ��Ƶ���㣬�漰�����Ʊ���������ԭ��Ӧ�ζ���֪ʶ�㣬����ʵ���ԭ����ʵ�������������������������ԭ�����ǽ��Ĺؼ���ע������������ã���Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ���ۼ� | �Ͽ��ü����յ����������ɸü��ų�������/kJ•mol-1 | ���ۼ� | �Ͽ��ü����յ����������ɸü��ų�������/kJ•mol-1 | ���ۼ� | �Ͽ��ü����յ����������ɸü��ų�������/kJ•mol-1 |
| H-H | 436 | H-Br | 366 | Cl-Cl | 243 |
| H-O | 463 | H-I | 298 | Br-Br | 193 |
| H-Cl | 432 | O=O | 496 | I-I | 151 |
| A�� | �٢� | B�� | �ڢ� | C�� | ֻ�Т� | D�� | ֻ�Т� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
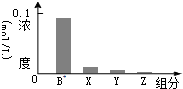 �����£�0.2mol/L��һԪ��HA���Ũ�ȵ�NaOH��Һ�������Ϻ�������Һ�в�������ּ�Ũ����ͼ��ʾ������˵����ȷ���� ��������
�����£�0.2mol/L��һԪ��HA���Ũ�ȵ�NaOH��Һ�������Ϻ�������Һ�в�������ּ�Ũ����ͼ��ʾ������˵����ȷ���� ��������| A�� | HAΪǿ�� | B�� | �û����Һ�У�c��A-��+c��Y��=c��Na+�� | ||
| C�� | ͼ��X��ʾHA��Y��ʾH+��Z��ʾOH- | D�� | �û��ҺpH=7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����Ŀǰ�����õ����������ɻ�ѧ��Ӧ������ | |
| B�� | ú��ʯ�͡���Ȼ���ǵ�����������Ҫ�����ֻ�ʯȼ�� | |
| C�� | �ҹ�Ŀǰ����Ҫ����Դ��ʯ�� | |
| D�� | �����仯�ǻ�ѧ��Ӧ�Ļ�������֮һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���˲����У�©���ļ��Ӧ�Ӵ��ձ��ڱ� | |
| B�� | �ò�����պȡCH3COOH��Һ����ˮʪ���pH��ֽ�ϣ��ⶨ����Һ��pH | |
| C�� | �к͵ζ�ʱ���ζ�������ʢװ�ķ�ӦҺ��ϴ2��3 �Σ� | |
| D�� | ��Һ�����У��²�Һ����¶˷ų����ϲ�Һ����Ͽڵ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
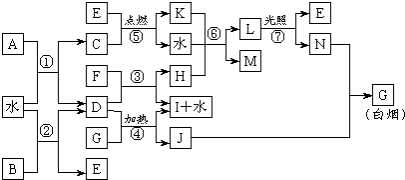
 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com