
 ���ڿ����о��õ���Ƭ5.0 gͶ��ʢ��500 mL 0.5 mol·L��1������Һ���ձ��У�����Ƭ�����ᷴӦ���������������뷴Ӧʱ��Ĺ�ϵ����
���ڿ����о��õ���Ƭ5.0 gͶ��ʢ��500 mL 0.5 mol·L��1������Һ���ձ��У�����Ƭ�����ᷴӦ���������������뷴Ӧʱ��Ĺ�ϵ����
��ͼ��ʾ��������������ʾ���ش��������⣺
������O��a�β�����������ԭ���û�ѧ����ʽ����Ϊ
________________________________________________��
������b��c�β����������������ӽϿ����Ҫԭ����
________________________________________________��
������Һ�м����������ʣ��ܼӿ�������ѧ��Ӧ���ʵ���
________________________________________________��
A������ˮB���������ۡ�C�������Ȼ�����Һ��D��Ũ���ᡡE����������ͭ��Һ
(2)��(8��)�������ʵ�����A��B�������2L���ܱ������У��������·�Ӧ��
3A(g)+B(g) ![]() xC(g)+2D(g),5min����c(D)=0.5 mol/L��c(A):c(B)=1:2,C�ķ�Ӧ������0.15 mol/(L•min)��
xC(g)+2D(g),5min����c(D)=0.5 mol/L��c(A):c(B)=1:2,C�ķ�Ӧ������0.15 mol/(L•min)��
��B�ķ�Ӧ����V(B)=
��X=
��A��5minĩ��Ũ����
��10min��������Ӧ������ȣ�����ѡ����һ����ȷ����Ӧ������ȵ��� ��
A.�����������ܶȲ���
B.������ѹǿ����
��1��������O��a�β�����������ԭ���û�ѧ����ʽ����Ϊ
_______Al2O3 + 6HCl = 2AlCl3 + 3H2O_________________��
������b��c�β����������������ӽϿ����Ҫԭ����
_______ ��Ӧ���ȣ�ʹ��Ӧ���ʼӿ�_________________________��
������Һ�м����������ʣ��ܼӿ�������ѧ��Ӧ���ʵ���
________B��D��E________________________________________��
(2)�� B�ķ�Ӧ����V(B)= 0.05 mol•L-1•min-1
��X= 3
��A��5minĩ��Ũ���� 1mol/L
��10min��������Ӧ������ȣ�����ѡ����һ����ȷ����Ӧ������ȵ��� B��C��D��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���ڿ����о��õ���Ƭ5.0gͶ��ʢ��500mL0��5mol?L-1������Һ���ձ��и���Ƭ�����ᷴӦ���������������뷴Ӧʱ�������ͼ��ʾ��������������ʾ���ش��������⣺
���ڿ����о��õ���Ƭ5.0gͶ��ʢ��500mL0��5mol?L-1������Һ���ձ��и���Ƭ�����ᷴӦ���������������뷴Ӧʱ�������ͼ��ʾ��������������ʾ���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2011?ӥ̶��ģ�����ڿ����о��õ���Ƭ5.0gͶ��ʢ�� 500mL 0.5mol?L-1������Һ���ձ��У�����Ƭ�����ᷴӦ��������������v�뷴Ӧʱ��t������ͼ��������������ʾ���������۴�����ǣ�������
��2011?ӥ̶��ģ�����ڿ����о��õ���Ƭ5.0gͶ��ʢ�� 500mL 0.5mol?L-1������Һ���ձ��У�����Ƭ�����ᷴӦ��������������v�뷴Ӧʱ��t������ͼ��������������ʾ���������۴�����ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
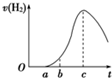 ���ڿ����о��õ���Ƭ5.0gͶ��ʢ��500mL 0.5mol?L-1������Һ���ձ��У�����Ƭ�����ᷴӦ���������������뷴Ӧʱ��Ĺ�ϵ������ͼ��������ʾ��
���ڿ����о��õ���Ƭ5.0gͶ��ʢ��500mL 0.5mol?L-1������Һ���ձ��У�����Ƭ�����ᷴӦ���������������뷴Ӧʱ��Ĺ�ϵ������ͼ��������ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ڿ����о��õ���Ƭ5.0 gͶ��ʢ�� 500mL0.5 mol��L-1������Һ���ձ��У�����Ƭ�����ᷴӦ���������������뷴Ӧʱ��������ҵ�������������ʾ���ش��������⣺
��1��������0��a�β�����������ԭ��___________,
�йط�Ӧ�����ӷ���ʽΪ____________ ��
��2��������a��b�β������������ʽ�����ԭ��___________
�йصĻ�ѧ����ʽ__________________________��
��3��������b��c�Σ������������������ӽϿ����Ҫԭ��_________________________��
��4��������c�Ժ����������������½�����Ҫԭ��_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��ɽ��ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��8�֣����ڿ����о��õ���Ƭ5.0 gͶ��ʢ��500 mL 0.5 mol��L��1������Һ���ձ��У�����Ƭ�����ᷴӦ���������������뷴Ӧʱ��Ĺ�ϵ������ͼ��������ʾ���ش��������⣺

(1)������O ��a�β�����������ԭ����____________________________________________________________��
�йط�Ӧ�Ļ�ѧ����ʽΪ
__________________________________________________��
(2)����a��c�Σ������������������ӽϿ����Ҫԭ����
________________________________________________________________________
________________________________________________________________________��
(3)������c�Ժ����������������½�����Ҫԭ����_________________________��
(4)�÷�Ӧ��ʹ�ô�������ʹH2����������______(��ᡱ���ᡱ)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com