
��17�֣�ij�л���A����C��H��O����Ԫ����ɣ���һ�������£���A����ת��Ϊ�л���B��C��D��E��ת���ϵ���£�
��֪D����Է�������Ϊ44�����ɷ���������Ӧ��
��1��д��D�����ƣ�  ��2��������C�Ľṹ��ʽΪ
��2��������C�Ľṹ��ʽΪ
��3��д��E��ͬ���칹��Ľṹ��ʽ
��4��д��ʵ������ת���Ļ�ѧ����ʽ��ע�������ͷ�Ӧ���ͣ��л���д�ṹ��ʽ����
A��B�� ����Ӧ���� ��
A��D�� ����Ӧ���� ��
A��E��F��H2O�� ����Ӧ���� ��
 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij�л���A����C��H��O����Ԫ����ɣ���һ����������A����ת��Ϊ�л���B��C��D��E��ת���ϵ���£�
ij�л���A����C��H��O����Ԫ����ɣ���һ����������A����ת��Ϊ�л���B��C��D��E��ת���ϵ���£�
| ||
| �� |
| Ũ���� |
| �� |
| Ũ���� |
| �� |
| ���� |
| ���� |
| ���� |
| �� |
| ���� |
| �� |
| Ũ���� |
| �� |
| Ũ���� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| �� |
| �� |
| �� |
| �� |
| ���� |
| ���� |
| Ũ���� |
| �� |
| Ũ���� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ũ���� |
| �� |
| Cu |
| �� |
| Ũ���� |
| �� |
| Cu |
| �� |
�鿴�𰸺ͽ���>>
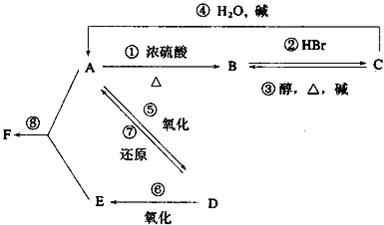
��Ŀ�����л�ѧ ��Դ��2010�꽭�պ�����ѧ��һ��ѧ����ĩ���Ի�ѧ ���ͣ�ʵ����
ij�л���A����C��H��O����Ԫ����ɣ���һ�������£�A��B��C��D��E��F��������ת����ϵ��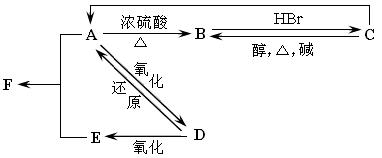
��֪D�������ܶ���������29���������Է���������Ӧ��
��1��д��F�Ľṹ��ʽ �� ��
��2��д��C �� A�ķ�Ӧ���� �� ��
��3��д��A �� D�ķ�Ӧ����ʽ �� ��
д��B �� C�ķ�Ӧ����ʽ �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014������ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��16�֣�ij�л���A����C��H��O����Ԫ����ɣ���һ�������£���A����ת��Ϊ�л���B��C��D��E��C�ֿ���ת��ΪB��A�����ǵ�ת����ϵ���£�
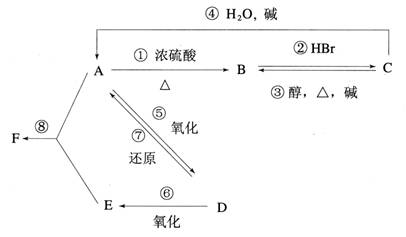
��֪D�������ܶ���������22���������Է���������Ӧ��
��A~F�Ľṹ��ʽ����Ϊ____________��____________��____________��____________��____________��____________��
���ڢ�~���ת����������ȥ��Ӧ����____________�ӳɷ�Ӧ����____________ȡ����Ӧ����____________��
�ǣ���A~F�У�ѡ���ʵ�����ĸ��գ�����������ʳƷ��װ���ĵ�����__________����������������Cu(OH)2��Ӧ����_______________��Ŀǰ�ᳫ���ں����Ͱ�һ���������������������ȼ�ϵ���________________��
��4���ֱ�д���٢ݵĻ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com