
| �ɷ� | Na+ | K+ | Ca2+ | Mg2+ | Cl- | SO42- | HCO3- |
| ����/mg•L-1 | 9360 | 83 | 160 | 1100 | 16000 | 1200 | 118 |
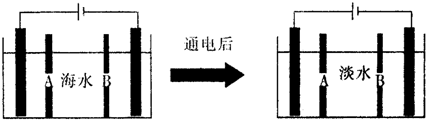
���� ��1��������ˮ�д��ڵ����������жϣ�̼���������������������ˮ���Լ��ԣ���Ϻ�ˮ�и����Ӻ���Ϊ200mg/L���������ʵ���Ũ�ȸ������õ���
��2�������ݵ�������������ˮʾ��ͼ�����������ӽ���Ĥ������������ͨ�����������������ӵõ����ӷ�����ԭ��Ӧ��
�������������ӵõ����ӷ�����ԭ��Ӧ��������������������Ũ������ˮ�к���̼��������ӡ������ӣ����������Ӻ�̼��������ӷ�Ӧ����̼�����������������ɰ�ɫ����̼��ƣ�
��3������ˮ�dz�ȥ�������ʣ�����ˮ�dz�ȥ��þ���ӣ��۱�ϩ�����DZ�ϩ���ƼӾ۷�Ӧ�IJ������Ϊ��ϩ���ƣ�
��4����Ӧ��Br2������������ԭ�����ã����ݵ���ת���غ��֪���������뻹ԭ�����ʵ���֮��Ϊ5��1���ݴ˼��㣮
��� �⣺��1����ˮ�д��ڵ����������жϣ�̼���������������������ˮ���Լ��ԣ����ӷ���ʽΪ��HCO3-+H2O?H2CO3+OH-����Ϻ�ˮ�и����Ӻ���Ϊ160mg/L�����ʵ���Ũ��=$\frac{\frac{160��1{0}^{-3}g}{40g/mol}}{1L}$=4��10-3 mol/L��
�ʴ�Ϊ��HCO3-+H2O?H2CO3+OH-��4��10-3 ��
��2�����������������ӵõ����ӷ�����ԭ��Ӧ�������ӷŵ��������������ӣ����Ե缫��ӦΪ��2H2O+2e-=H2��+2OH-��2H++2e-=H2���������ӽ���Ĥ��ָ����װ����Bһ�˽���Ĥ��
�ʴ�Ϊ��B��
���������Ǻ�ˮ�е�������ʧ���ӷ���������Ӧ�����������缫��ӦΪ��2Cl--2e-=Cl2���������������ӵõ����ӷ�����ԭ��Ӧ��������������������Ũ������ˮ�к���̼��������ӡ������ӣ����������Ӻ�̼��������ӷ�Ӧ����̼�����������������ɰ�ɫ����̼��ƣ�����̼��Ƴ��������ӷ���ʽΪ��Ca2++OH-+HCO3-=CaCO3��+H2O��
�ʴ�Ϊ��2Cl--2e-=Cl2����Ca2++OH-+HCO3-=CaCO3��+H2O��
��3��ˮ�ľ����dz�ȥ���������ʣ�һ��������ˮ�����ɵ��������������������������ʽ��о�ˮ��ˮ�������ǽ���Ca2+��Mg2+��Ũ�ȣ������ܶ࣬�����ӽ�������ʯ�Ҵ���ȣ��۱�ϩ�����DZ�ϩ���ƼӾ۷�Ӧ�IJ������Ϊ��ϩ���ƣ��ṹ��ʽΪ��CH2=CHCOONa��
�ʴ�Ϊ��ˮ�ľ�����ͨ�����������������ȣ���ˮ�еĽ��弰�������ȥ����ˮ��������ʹˮ�е�Ca2+��Mg2+Ũ�ȼ�С��CH2=CHCOONa��
��4����Ӧ��Br2������������ԭ�����ã����ݵ���ת���غ��֪��2��n��������Br2��=2��5��n��ԭ����Br2������n��������Br2����n��ԭ����Br2��=5��1��������80gBr2���ʵ���=$\frac{80g}{160g/mol}$=0.5mol��1mol Br2ʱ��ת�Ƶĵ�����Ϊ1mol��2��$\frac{1}{1+5}$��5=$\frac{5}{3}$mol��������0.5mol Br2ʱ��ת�Ƶĵ�����Ϊ$\frac{5}{6}$mol
�ʴ�Ϊ��$\frac{5}{6}$��
���� ���⿼���˺�ˮ��Դ�����ã���Ҫ�ǵ��ԭ���ķ����͵缫��Ӧ��д������ˮ�⡢������ԭ��Ӧ����ת�Ƶļ���ȣ����ջ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
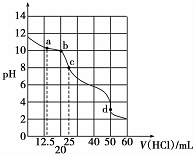 �ڳ����£�0.1000mol•L-1Na2CO3��Һ25mL��0.1000mol•L-1����ζ�����ζ�������ͼ��ʾ���Եζ�������������Һ���������Ũ�ȼ�Ĺ�ϵ�������й�˵����ȷ���ǣ�������
�ڳ����£�0.1000mol•L-1Na2CO3��Һ25mL��0.1000mol•L-1����ζ�����ζ�������ͼ��ʾ���Եζ�������������Һ���������Ũ�ȼ�Ĺ�ϵ�������й�˵����ȷ���ǣ�������| A�� | a�㣺c��CO32-��=c��HCO3-����c��OH-�� | B�� | b�㣺5c��Cl-����4c�� HCO3-��+4c��CO32-�� | ||
| C�� | c�㣺c�� OH -��=c��H+��+c��HCO3-��+2c��H2CO3�� | D�� | d�㣺c��H+��=c��CO32-��+c�� HCO3-��+c��OH -�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
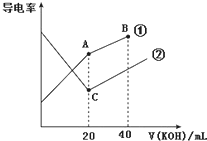 �絼���Ǻ����������Һ����������С��������������Һ�絼�ʱ仯����ȷ���ζ���Ӧ���յ㣮��ͼ��ijͬѧ��0.1mol•L-1 KOH��Һ�ֱ�ζ������Ϊ20mL��Ũ�Ⱦ�Ϊ0.1mol•L-1��HCl��CH3COOH��Һ�ζ�����ʾ��ͼ�������Һ����仯���Բ��ƣ��������й��жϲ���ȷ���ǣ�������
�絼���Ǻ����������Һ����������С��������������Һ�絼�ʱ仯����ȷ���ζ���Ӧ���յ㣮��ͼ��ijͬѧ��0.1mol•L-1 KOH��Һ�ֱ�ζ������Ϊ20mL��Ũ�Ⱦ�Ϊ0.1mol•L-1��HCl��CH3COOH��Һ�ζ�����ʾ��ͼ�������Һ����仯���Բ��ƣ��������й��жϲ���ȷ���ǣ�������| A�� | ���ߢٴ���0.1 mol•L-1 KOH��Һ�ζ�CH3COOH��Һ�ĵζ����� | |
| B�� | ����ͬ�¶��£�C��ˮ�����c��H+������A��ˮ�����c��H+�� | |
| C�� | ��A�����Һ���У�c��CH3COO-��+c��OH-��-c��H+���T0.05 mol•L-1 | |
| D�� | ��B�����Һ���У�c��K+����c��OH-����c��CH3COO-����c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
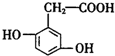 ��������˵��������ǣ�������
��������˵��������ǣ�������| A�� | 1mol�������������Ũ��ˮ��Ӧ���������3mol Br2 | |
| B�� | 1mol������������4mol H2��Ӧ | |
| C�� | ������������ͬһƽ���ϵ�̼ԭ��������7�� | |
| D�� | ��������̼��������Һ��Ӧ�ų�CO2������2.24LCO2������£���Ҫ�����16.8g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
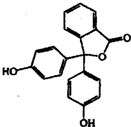 �������ڱ�����������
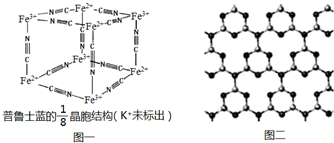
�������ڱ����������� ����һ�������·�Ӧ���Ƶ÷�̪����̪�ķ��ӽṹ��ͼ��ʾ������˵����ȷ���ǣ�������
����һ�������·�Ӧ���Ƶ÷�̪����̪�ķ��ӽṹ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | �����Ʒ�̪�ķ�Ӧ����ȡ����Ӧ | |
| B�� | ��̪�����е�̼ԭ���п��ܹ�����ͬһƽ�� | |
| C�� | ����NaOH��Һ��Ӧ��1mol��̪��������4 molNaOH | |
| D�� | ����̪���ھƾ���ɵķ�̪�Լ����������Ի�����ˮ��Һ�п��ܻ���ְ�ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��ѧʽ | ���볣�� |
| CH3COOH | Ka=1.76��10-5 |
| H2SO3 | ${K_{a_1}}$=1.54��10-2 |
| ${K_{a_2}}$=1.02��10-7 | |
| HF | Ka=6.03��10-4 |
| A�� | 1mol•L-1NaHA��Һ��һ�����ڣ�c��Na+��=c��H2A��+c��HA-��+c��A2-�� | |
| B�� | ���������Һ�м����������ᣬ�õ������Ի����Һ�У�c��Na+����c��CH3COO-����c��H+����c��OH-�� | |
| C�� | pH������3�Ĵ�����������Һ�������Ϻ���Һ��pH��� | |
| D�� | ��֪ij�¶��³�������ĵ���ƽ�ⳣ���������ͬ���ʵ���Ũ�ȵ�CH3COONa��NaF��Na2SO3��NaHSO3ˮ��Һ����Һ������������С�������е�˳����Na2SO3��CH3COONa��NaF��NaHSO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ����� | ����ˮ����� | KI��Һ����� | ���ĵ�Na2S2O3��Һ����� |
| 1 | 10.00mL | 10.00mL | 19.96mL |
| 2 | 10.00mL | 10.00mL | 20.04mL |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com