
��չ��϶�������ʵʩ���ܼ��ŵ���Ҫ��ʩ֮һ����϶������ĵ綯��Ŀǰһ��ʹ�õ��������ء��������»����ʱ���綯���ṩ�ƶ��������������͵����ģ���ɲ��������ʱ�綯�����ڳ��״̬�Խ�ʡ�ܺġ������ز������Ļ�����Ϊ�����������������M��ʾ��Ϊ��������Һ����ҪΪKOH��Ϊ���Һ�������س�ŵ�ԭ����ͼ�����ܷ�ӦʽΪ�� H2+2NiOOH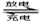 2Ni(OH)2
2Ni(OH)2
�����йػ�϶��������ж���ȷ���ǣ�������
A.�����»����ʱ��ÿ����22.4LH2���ӵ缫������缫�ҵĵ�����2mol
B�������»����ʱ���ҵ缫��Χ��Һ��pH����С
C����ɲ��������ʱ����Һ�е�K+���ҵ缫Ǩ��
D����ɲ��������ʱ���缫�ĵ缫��ӦʽΪ2H2O+2e-=H2��+2OH-
��֪ʶ�㡿��ѧ��Դ���͵��
���𰸽�����D ������A�������»����ʱ���缫�缫��ӦʽΪH2+2OH-+2e-═2H2O
ÿ����22.4LH2���ӵ缫������缫�ĵ�����2mol����A����B�������»����ʱ����ԭ��ع���ԭ�����ҵ缫Ϊ��������NiOOHת��Ϊ���������Ĺ��̣��ҵ缫��Χ�����������ӵ�Ũ������pH������B����C����ɲ��������ʱ�ǵ��صĹ���ԭ�����缫����������Һ�е�K+��缫Ǩ�ƣ���C����D����ɲ��������ʱ��ش��ڳ��״̬�����صĹ���ԭ�����缫�������������ӷ����õ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ2H2O+2e-═H2��+2OH-����D��ȷ��
�ʴ�ѡD
��˼·�㲦�����⿼��ѧ��ԭ��غ͵��صĹ���ԭ��֪ʶ��ע��ƽʱ֪ʶ�Ļ����Լ�����֪ʶ�����գ��Ѷ��еȡ�


 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
1.52gͭþ�Ͻ���ȫ�ܽ���50mL�ܶ�Ϊ1.40g/mL����������Ϊ63%��Ũ�����У��õ�NO2��N2O4 �Ļ������1120mL (��״��)����Ӧ�����Һ�м���1.0mol/LNaOH��Һ,����������ȫ������ʱ���õ�2.54g����������˵������ȷ���� �� ��
A.�úϽ���ͭ��þ�����ʵ���֮����2 ︰1
B.��Ũ������HNO3�����ʵ���Ũ����14.0mol/L
C. NO2��N2O4 �Ļ�������У�NO2 �����������80%
D. �õ�2.54����ʱ������NaOH��Һ�������600mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������ʼ��仯�����ڹ�ҵ���й㷺Ӧ�á�
��1��ͬ��ʯ�ڸ������Ʊ������Ȼ�ѧ����ʽΪ��
4Ca5(PO4)3F(s)+21SiO2(s)+30C(s)=3P4(g)+20CaSiO3(s)+30CO(g)+SiF4(g) H
H
��֪��ͬ�����£�
4Ca3(PO4)2F(s)+3SiO2(s)=6Ca3(PO4)2(s)+2CaSiO3(s)+SiF4(g) ��H1
2Ca3(PO4)2(s)+10C(s)=P4(g)+6CaO(s)+10CO(g) ��H2
SiO2(s)+CaO(s)=CaSiO3(s) ��H3
�á�H1����H2�͡�H3��ʾ H����
H���� H=____________________��
H=____________________��
��2�������������Ϊ����������ӣ�����ṹʽ��ͼ��֮����ȥ����ˮ���Ӳ����ṹʽΪ________________________________�����������ƣ��׳ơ����ơ����dz��õ�ˮ���������仯ѧʽΪ____________��
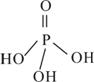
��3���������ƣ�NaH2PO2�������ڹ�ҵ�ϵĻ�ѧ������
�ٻ�ѧ��������Һ�к���Ni2+��H2PO2���������Ե������·���������Ӧ��
��a��_____ Ni2+ + ____ H2PO2��+ _____ �� ___Ni++______ H2PO3��+ ____
��b��6H2PO-2 +2H+ =2P+4H2PO3+3H2��
���������д������ƽ��Ӧʽ��a����
�����â��з�Ӧ�������϶Ƽ����������—�Ͻ𣬴Ӷ��ﵽ��ѧ������Ŀ�ģ�����һ�ֳ����Ļ�ѧ�ơ�������·���Ƚϻ�ѧ�����ơ�
�����ϵIJ�ͬ�㣺______________________________________________________��
ԭ���ϵIJ�ͬ�㣺______________________________________________________��
��ѧ�Ƶ��ŵ㣺________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ1���ڽ���п��������һ����ij��Һ��ʪ����ֽ��ͼ2��NaBH4/H2O2ȼ�ϵ�أ������е�˵������ȷ���� �� ��

A��ͼ2����ڷŵ�����У��������ĵ缫��ӦΪ��BH4-�� 8e- + 8OH-��BO2- + 6H2O
B������ϡ�����ʪ��ֽ���õ��߽�a��b���������е��Ӵ�b������ͨ����������a��
C�����������ƺ���ɫʯ��Ļ����Һ��ʪ��ֽ���õ��߽�a��b��������ɿ���Ǧ��оC�㴦������ɫʯ�����ֽ�������˴������缫��ӦΪ��O2 + 2H2O+ 4e- = 4OH-
D������KI-������Һ��ʪ��ֽ��ͬʱ�õ��߽�a��b�ֱ���A��B�缫������������Ǧ��оC�㴦���ֱ���������b�����ӵ���ȼ�ϵ�ص�A��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
SO2��NO�Ǵ�����Ⱦ�����SO2 ��NO�����Na2S2O4��NH4NO3��Ʒ������ͼ���£�CeΪ��Ԫ�أ���
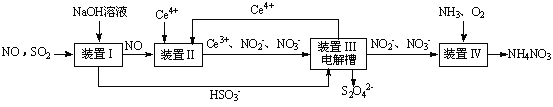
��1��װ�â�������HSO3�������ӷ���Ϊ ��
��2�����������H2SO3��HSO3����SO32����������SO2��NaOH��Һ��Ӧ�����Һ�У����ǵ����ʵ�������X(i)����ҺpH �Ĺ�ϵ����ͼ��ʾ��
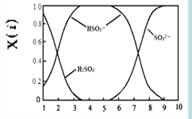
������˵����ȷ���� ������ĸ��ţ���
a��pH=8ʱ����Һ��c(HSO3��) < c(SO32��)
b��pH=7ʱ����Һ��c(Na+) =c(HSO3��)+c(SO32��)
c��Ϊ��þ����ܴ���NaHSO3���ɽ���Һ��pH������4��5����
����pH=5��NaHSO3��Һ�еμ�һ��Ũ�ȵ�CaCl2��Һ����Һ�г��ֻ��ǣ�pH��Ϊ2���û�ѧƽ���ƶ�ԭ��������ҺpH���͵�ԭ�� ��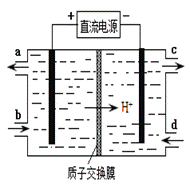
��3��װ�â��У����������£�NO��Ce4+�����IJ�����Ҫ��NO3����NO2����д������NO3�������ӷ���ʽ ��
��4��װ�â������֮һ������Ce4+����ԭ������ͼ��ʾ��
������Ce4+�ĵ缫��ӦʽΪ ��
������Ce4+�ӵ��۵� ������ĸ��ţ���������
��5����֪����װ�â�����Һ�У�NO2����Ũ��Ϊa g·L-1��Ҫʹ1 m3����Һ�е�NO2����ȫת��ΪNH4NO3����������װ�â���ͨ���״���µ�O2 L�����ú�a����ʽ��ʾ������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪������KMnO4�����еμ�Ũ���ᣬ��������ɫ���壻����FeCl2��Һ��ͨ������ʵ��ٲ��������壬��Һ���ɫ����ȡ����ʵ������ɵ���Һ���ڵ���KI��ֽ�ϣ���ֽ����ɫ��
�����ж���ȷ����(����)
A������ʵ��֤�������ԣ�MnO4��>Cl2>Fe3��>I2
B������ʵ���У���������������ԭ��Ӧ
C��ʵ������ɵ����岻��ʹʪ��ĵ���KI��ֽ����
D��ʵ���֤��Fe2���������������л�ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ��Na2O2��SO2��Na2SO4��Ƚϣ�Na2O2��������ͬ����
A��2Na2O2��2CO2��2Na2CO3��O2 B��2Na2O2��2SO3��2Na2SO4��O2
C��2Na2O2��H2SO4��Na2SO4��H2O2 D��3Na2O2��Cr2O3��2Na2CrO4��Na2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
С��Ϊ��֤NO2�������Ժ�NO�Ļ�ԭ�ԣ����������װ����ȡNO2��NO������֤�����ʣ�װ��ͼ���£�
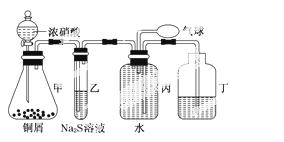
(1)д�����з�Ӧ�����ӷ���ʽ��______________________________________________��
���е�������____________����֤��NO2�������ԣ��ڱ��й��������������__________����֤��NO�Ļ�ԭ�ԣ�
(2)ʵ��ǰ���г���ˮ��������_____________________________________________
________________________________________________________________________(�÷�Ӧ����ʽ�ͼ�Ҫ���ֻش�)��
(3)С����С����ʵ�������������ɣ�����Ϊ���е���������֤��NO2�������ԣ�����������___________________________________________________________________
________________________________________________________________________.
����Ϊ��������ȷ֤��NO2�������ԣ�_____________________________________
________________________________________________________________________
________________________________________________________________________(��Ҫ�ش��ԭ��������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮ�в������зḻ �ķǽ���Ԫ����Դ(���ȡ��塢���)�������зḻ�Ľ���Ԫ����Դ(��Na��Mg��Fe��Cr��)��
�ķǽ���Ԫ����Դ(���ȡ��塢���)�������зḻ�Ľ���Ԫ����Դ(��Na��Mg��Fe��Cr��)��
(1)��ˮɹ�ε�ԭ���ǣ�________��д���Ȼ��Ƶĵ���ʽ��________����Na��Clͬ���ڣ��Ҽ����Ӱ뾶��С�����ӽṹʾ��ͼ��________��
(2)ɹ���Ĵ��γ�����MgSO4��CaSO4�����ʣ�Ϊ�˵õ������Σ����ᴿ���̲�������ͼ����Լ���˳���ǣ����ܽ⣬��________���ۼӹ���Na2CO3��Һ����________���ݹ��˳�ȥ���ʣ���________���������ᾧ��
(3)ɹ�εõ���ĸҺ(��±)�к��зḻ��þԪ�أ������г�����Fe2����Cr3���ȣ�Ϊ����þʹ��ת��ΪMgCl2���壬�����ȥ��Щ�������ӡ�
�й����ϣ�
| M(OH) | pH | |
| ��ʼ���� | ������ȫ | |
| Fe(OH)2 | 7.6 | 9.6 |
| Fe(OH)3 | 2.7 | 3.7 |
| Mg(OH)2 | 9.5 | 11.0 |
| Cr(OH)3 | 4.3 | 5.0 |
Ϊ����Ч��ȥ�������ӣ��ֲ������µ��� �����ӣ��������㡰��ɫ��ѧ�����������Լ�ѡ��Ͳ����ǣ�
�����ӣ��������㡰��ɫ��ѧ�����������Լ�ѡ��Ͳ����ǣ�
���ȼ�________��Ŀ����________��
���ټ�________��Ŀ����________��
�۹��˺�Ϊ�ܵõ�������MgCl2���壬���õIJ��������ǣ�
_______________ __________________________________
__________________________________
__________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com