
(16��) �±�ΪԪ�����ڱ���һ���֣������Ԫ�آ٣����ڱ��е�λ�ã��û�ѧ����
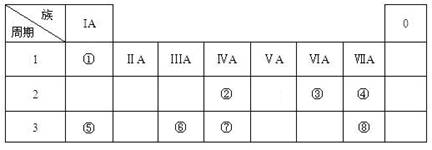
�ش��������⣺
��1���ܡ��ݡ���ԭ�Ӱ뾶�ɴ�С��˳��Ϊ_______________________���ڡ��ߡ������ۺ������������ǿ������˳����______________________��
��2���ɱ�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬�������ϡҺ�ױ����ֽ⣬��ʹ�õĴ���Ϊ������ţ�_________________��
a��MnO2������������ b��FeCl 3���������� c��Na2SO3���������� d��KMnO4
����֪1 �˸�Һ̬������ֽ�ɢ۵ĵ��ʺ�һ�ֳ���Һ��ʱ���ɷų�2.9kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��
��3����ҵ�ϳ����õ��A�ͱ���ʯ(Na3AlF6)�����ķ���ұ���Ʊ��ĵ��ʣ����A��������ͺ��������������ǶȽ��ͼӱ���ʯ(Na3AlF6)��ԭ�� �� ��д�����ʱ�ĵ缫��Ӧʽ�� ��
��4���ס��ҡ�������������Ԫ����ɵ�˫ԭ�ӷ��ӻ���˫ԭ�������ӣ��Ҽס��ҡ����ĵ���������ȡ�����һ�ּ�ǿ�������Ե��ʡ�����ݵ������ӿ��γ�һ�ֵ���ɫ����B���ù����ˮ��Ӧ�ɵõ��۵ĵ��ʡ���B����ʽ ���ҵĽṹʽ �������Ԫ�ص�ԭ�ӽṹʾ��ͼ ����֤�������Ԫ�طǽ����Ժ�ǿ����ʵ ��(�ξ�һ������)
������Na��Al�� F�� HClO4��H2CO3��H2SiO3
��2�� a b 2H2O2��l����2H2O��l��+O2(g) ��H����197.2kJ��mol��1
��3��������Ϊ���Ӿ��壬�ƻ����Ӽ���Ҫ�϶���������ӱ���ʯ�ܽ����۵�
������2O2����4e-=O2 ���� Al3++3e��=Al
��4�� 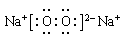 H��Cl
H��Cl 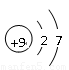 ��H2�ڰ������ܻ��ϻ�HF���ȶ�
��H2�ڰ������ܻ��ϻ�HF���ȶ�
���������������ڱ��Ľṹ��Ԫ�������ɵ�Ӧ�á�����Ԫ�������ڱ��е����λ�ÿ��Եó���������Ԫ�أ���̼Ԫ�أ�����Ԫ�أ��ܷ�Ԫ�أ�����Ԫ�أ�����Ԫ�أ��߹�Ԫ�أ�����Ԫ�أ�����Ԫ�ء�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(16��) �±�ΪԪ�����ڱ���һ���֣������Ԫ�آ٣����ڱ��е�λ�ã��û�ѧ����
�ش��������⣺
��1���ܡ��ݡ���ԭ�Ӱ뾶�ɴ�С��˳��Ϊ_______________________���ڡ��ߡ������ۺ������������ǿ������˳����______________________��
��2���ɱ�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬�������ϡҺ�ױ����ֽ⣬��ʹ�õĴ���Ϊ������ţ�_________________��
a��MnO2������������ b��FeCl 3���������� c��Na2SO3���������� d��KMnO4
����֪1 �˸�Һ̬������ֽ�ɢ۵ĵ��ʺ�һ�ֳ���Һ��ʱ���ɷų�2.9kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��
��3����ҵ�ϳ����õ��A�ͱ���ʯ(Na3AlF6)�����ķ���ұ���Ʊ��ĵ��ʣ����A��������ͺ��������������ǶȽ��ͼӱ���ʯ(Na3AlF6)��ԭ�� �� ��д�����ʱ�ĵ缫��Ӧʽ�� ��
��4���ס��ҡ�������������Ԫ����ɵ�˫ԭ�ӷ��ӻ���˫ԭ�������ӣ��Ҽס��ҡ����ĵ���������ȡ�����һ�ּ�ǿ�������Ե��ʡ�����ݵ������ӿ��γ�һ�ֵ���ɫ����B���ù����ˮ��Ӧ�ɵõ��۵ĵ��ʡ���B����ʽ ���ҵĽṹʽ �������Ԫ�ص�ԭ�ӽṹʾ��ͼ ����֤�������Ԫ�طǽ����Ժ�ǿ����ʵ ��(�ξ�һ������)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ������ѧ��һ��ѧ�ڵ�һ�ν��Բ��Ի�ѧ�Ծ����������� ���ͣ������
(16��)�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ٣����ڱ��е�λ�ã��û�ѧ����
�ش��������⣺
��1���ܡ��ݡ���ԭ�Ӱ뾶�ɴ�С��˳��Ϊ_______________________���ڡ��ߡ������ۺ������������ǿ������˳����______________________��
��2���ɱ�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬�������ϡҺ�ױ����ֽ⣬��ʹ�õĴ���Ϊ������ţ�_________________��
a��MnO2������������b��FeCl 3���������� c��Na2SO3���������� d��KMnO4
����֪1 �˸�Һ̬������ֽ�ɢ۵ĵ��ʺ�һ�ֳ���Һ��ʱ���ɷų�2.9kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��
��3����ҵ�ϳ����õ��A�ͱ���ʯ(Na3AlF6)�����ķ���ұ���Ʊ��ĵ��ʣ����A��������ͺ��������������ǶȽ��ͼӱ���ʯ(Na3AlF6)��ԭ�� �� ��д�����ʱ�ĵ缫��Ӧʽ�� ��
��4���ס��ҡ�������������Ԫ����ɵ�˫ԭ�ӷ��ӻ���˫ԭ�������ӣ��Ҽס��ҡ����ĵ���������ȡ�����һ�ּ�ǿ�������Ե��ʡ�����ݵ������ӿ��γ�һ�ֵ���ɫ����B���ù����ˮ��Ӧ�ɵõ��۵ĵ��ʡ���B����ʽ ���ҵĽṹʽ �������Ԫ�ص�ԭ�ӽṹʾ��ͼ ����֤�������Ԫ�طǽ����Ժ�ǿ����ʵ ��(�ξ�һ������)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012���������ϴ�ѧ���и߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
(16��)�±�Ϊ���ڱ���һ���֣��������е���ĸ�ֱ����һ�ֻ�ѧԪ�ء�
�û�ѧ����ش��������⣺
(1)д��Ԫ��f�Ļ�̬ԭ�Ӻ�������Ų�ʽ___________________________��
(2)��c6a6�����У�Ԫ��cΪ �ӻ����÷����� ����(����ԡ��Ǽ��ԡ�)��
(3)ci2���ӵĵ���ʽΪ_________________________��ci2��ce2�Ƚϣ��е�ϸߵ���_____________(д����ʽ)��
(4)��һ�����ܣ�h______i���縺�ԣ�g______b(�����������������)��
(5)���й���Ԫ����Ԫ�����ڱ��е�λ���Լ�Ԫ��ԭ�ӵ���Χ�����Ų��ص���й�����ȷ�� ��
| A��jλ��Ԫ�����ڱ��е������ڡ���B�壬����ds��Ԫ�� |
| B��d�Ļ�̬ԭ���У�2p�ܼ�Ϊ�����������p��Ԫ�� |
| C�����������Ų�ʽΪ4s1��һ�����ڢ�A�� |
| D�����������Ų�ʽΪns2np1����Ԫ�ؿ����Ǣ�A����B�� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���Ĵ�ʡ�ɶ�����������9��ͳһ��⻯ѧ�Ծ��������棩 ���ͣ������
(13��) �±�ΪԪ�����ڱ���һ���֣����б�Ŵ�����Ӧ��Ԫ�ء�

��ش��������⣺
��1��д��Ԫ�آߵĻ�̬ԭ���Ų�ʽ ��Ԫ�آ�λ�� ����
��Ԫ��ԭ�ӵļ۲���ӵĹ����ʾʽΪ ��
��2����Ԫ�آ٢ۢ��γɵ�ij�����������ԣ����ⶨ������Ԫ�ص�������Ϊ1��6��16���û����������������ܶ�Ϊ23�������������ӻ�ԭ�ӵ��ӻ���ʽ�ֱ�Ϊ �� ��
��3��Ԫ�آܺ͢ĵ�һ�����ܴ�С˳���� > ����Ԫ�ط��ű�ʾ������д���ɢܺ͢�����Ԫ���γɵ���N3-��Ϊ�ȵ���������ӵĻ�ѧʽ ����VSEPR����Ϊ ��
��4���ڲⶨ�ٺ͢��γɵĻ��������Է�������ʱ��ʵ����ֵһ���������ֵ������Ҫԭ���� ��
��5���ܺ͢��γ�ij�ֻ�����ľ����ṹ����ͼ��ʾ��ÿ�������ʾ1��ԭ�ӣ����Т���-3�ۣ������仯ѧʽΪ ��

��ij������Ӻܵ͢�����⻯���γɵ������ӵ�����Ϊ ��
��ˮ��Һ��ɫΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com