
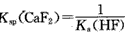

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 £Ø2012?Ōę×ƶžÄ££©ĪļÖŹŌŚĖ®ČÜŅŗÖŠµÄŠŠĪŖŹĒ֊ѧ»ÆѧµÄÖŲŅŖÄŚČŻ£®ŅŃÖŖĻĀĮŠĪļÖŹµÄµēĄė³£ŹżÖµ£Ø25”ę£©£ŗ
£Ø2012?Ōę×ƶžÄ££©ĪļÖŹŌŚĖ®ČÜŅŗÖŠµÄŠŠĪŖŹĒ֊ѧ»ÆѧµÄÖŲŅŖÄŚČŻ£®ŅŃÖŖĻĀĮŠĪļÖŹµÄµēĄė³£ŹżÖµ£Ø25”ę£©£ŗ
| ||
| ||
| 10-9 |
| x-10-2 |
| 10-9 |
| x-10-2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğø£½ØŹ”ÕÄÖŻŹŠÜ¼³Ē֊ѧø߶žÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
°“ŅŖĒó»Ų“š£ŗ
£Ø1£©Š“³öµēĄė·½³ĢŹ½£ŗ
NaHCO3_______________________________________________________________________
H2S__________________________________________________________________________
£Ø2£©Š“³öĖ®½āµÄĄė×Ó·½³ĢŹ½£ŗ
Na2CO3________________________________________________________________________
£Ø3£©ÓĆpHŹŌÖ½²ā¶Ø0.1 mol”¤L£1µÄ“æ¼īČÜŅŗµÄpH£¬ĘäÕżČ·µÄ²Ł×÷ŹĒ
__________________________________________________________________________________ӣ
£Ø4£©ĀČ»ÆĀĮĖ®ČÜŅŗ³Ź ŠŌ£ØĢīĖįŠŌ”¢ÖŠŠŌ”¢¼īŠŌ£©£¬ŌŅņŹĒ£ØÓĆ»Æѧ·½³ĢŹ½±ķŹ¾£©£ŗ_____________________ _______________________________________________ ______ ”£°ŃFeCl3ČÜŅŗÕōøÉ£¬×ĘÉÕ£¬×īŗóµĆµ½µÄÖ÷ŅŖ¹ĢĢå²śĪļŹĒ ”£
£Ø5£©ŹµŃéŹŅŌŚÅäÖĘAgNO3µÄČÜŅŗŹ±£¬³£½«AgNO3¹ĢĢåĻČČÜÓŚ½ĻÅصÄĻõĖįÖŠ£¬Č»ŗóŌŁÓĆÕōĮóĖ®Ļ”ŹĶµ½ĖłŠčµÄÅØ¶Č£¬ŅŌ £ØĢī”°“Ł½ų”±”¢”°ŅÖÖĘ”±£©Ag+Ė®½ā”£
£Ø6£©ŌŚ25”ęĻĀ£¬½«a mol”¤L£1µÄ°±Ė®Óė0.01 mol”¤L£1µÄŃĪĖįµČĢå»ż»ģŗĻ£¬³ä·Ö·“Ó¦ŗóČÜŅŗ³ŹÖŠŠŌ£¬Ōņ·“Ó¦ŗóČÜŅŗ“ęŌŚµÄĄė×ÓÓŠ £¬ĘäÅضČÓɓ󵽊”µÄĖ³ŠņĪŖ
£¬°±Ė®µÄÅضČa 0.01 mol”¤L£1£ØĢī”°£¾”±”¢”°£¼”±»ņ”°=”±£©”£
£Ø7£©ŌŚ25”ęĻĀ£¬ĻņÅØ¶Č¾łĪŖ0.20 mol”¤L£1µÄMgCl2ŗĶCuCl2»ģŗĻČÜŅŗÖŠÖšµĪ¼ÓČė°±Ė®£¬ĻČÉś³É ³Įµķ£ØĢī»ÆѧŹ½£©£¬Éś³ÉøĆ³ĮµķµÄĄė×Ó·½³ĢŹ½ĪŖ £»µ±²āµĆČÜŅŗpH=11Ź±£¬Ōņ“ĖĪĀ¶ČĻĀ²ŠĮōŌŚČÜŅŗÖŠµÄc(Mg2+)£ŗc(Cu2+)= £ØŅŃÖŖ25”ꏱKsp[Mg(OH)2]=1.8”Į10£11£¬Ksp[Cu(OH)2]=2.0”Į10£20£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģø£½ØŹ”ÕÄÖŻŹŠø߶žÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
°“ŅŖĒó»Ų“š£ŗ
£Ø1£©Š“³öµēĄė·½³ĢŹ½£ŗ
NaHCO3_______________________________________________________________________
H2S__________________________________________________________________________
£Ø2£©Š“³öĖ®½āµÄĄė×Ó·½³ĢŹ½£ŗ
Na2CO3________________________________________________________________________
£Ø3£©ÓĆpHŹŌÖ½²ā¶Ø0.1 mol”¤L£1µÄ“æ¼īČÜŅŗµÄpH£¬ĘäÕżČ·µÄ²Ł×÷ŹĒ
__________________________________________________________________________________ӣ
£Ø4£©ĀČ»ÆĀĮĖ®ČÜŅŗ³Ź ŠŌ£ØĢīĖįŠŌ”¢ÖŠŠŌ”¢¼īŠŌ£©£¬ŌŅņŹĒ£ØÓĆ»Æѧ·½³ĢŹ½±ķŹ¾£©£ŗ_____________________ _______________________________________________ ______ ”£°ŃFeCl3ČÜŅŗÕōøÉ£¬×ĘÉÕ£¬×īŗóµĆµ½µÄÖ÷ŅŖ¹ĢĢå²śĪļŹĒ ”£
£Ø5£©ŹµŃéŹŅŌŚÅäÖĘAgNO3µÄČÜŅŗŹ±£¬³£½«AgNO3¹ĢĢåĻČČÜÓŚ½ĻÅصÄĻõĖįÖŠ£¬Č»ŗóŌŁÓĆÕōĮóĖ®Ļ”ŹĶµ½ĖłŠčµÄÅØ¶Č£¬ŅŌ £ØĢī”°“Ł½ų”±”¢”°ŅÖÖĘ”±£©Ag+Ė®½ā”£
£Ø6£©ŌŚ25”ęĻĀ£¬½«a mol”¤L£1µÄ°±Ė®Óė0.01 mol”¤L£1µÄŃĪĖįµČĢå»ż»ģŗĻ£¬³ä·Ö·“Ó¦ŗóČÜŅŗ³ŹÖŠŠŌ£¬Ōņ·“Ó¦ŗóČÜŅŗ“ęŌŚµÄĄė×ÓÓŠ £¬ĘäÅضČÓɓ󵽊”µÄĖ³ŠņĪŖ
£¬°±Ė®µÄÅضČa 0.01 mol”¤L£1£ØĢī”°£¾”±”¢”°£¼”±»ņ”°=”±£©”£
£Ø7£©ŌŚ25”ęĻĀ£¬ĻņÅØ¶Č¾łĪŖ0.20 mol”¤L£1µÄMgCl2ŗĶCuCl2»ģŗĻČÜŅŗÖŠÖšµĪ¼ÓČė°±Ė®£¬ĻČÉś³É ³Įµķ£ØĢī»ÆѧŹ½£©£¬Éś³ÉøĆ³ĮµķµÄĄė×Ó·½³ĢŹ½ĪŖ £»µ±²āµĆČÜŅŗpH=11Ź±£¬Ōņ“ĖĪĀ¶ČĻĀ²ŠĮōŌŚČÜŅŗÖŠµÄc(Mg2+)£ŗc(Cu2+)= £ØŅŃÖŖ25”ꏱKsp[Mg(OH)2]=1.8”Į10£11£¬Ksp[Cu(OH)2]=2.0”Į10£20£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗŌę×ƶžÄ£ ĢāŠĶ£ŗĪŹ“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012ÄźÉ½¶«Ź”Ōę×ÆŹŠøßæ¼»Æѧ¶žÄ£ŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com