
��2011?������ģ��ijЩ������Һ�нᾧʱ�������ľ����ǽᾧˮ����±���¼��t���4����ͬ������ͭ��Һ�м������ˮ����ͭ�������Լ�����������ͭ���壨CuSO4?5H2O�����������¶�ά�ֲ��䣩��ʵ�����ݣ�
|
| 8.00g-5.00g |
| 10.0g-4.60g |
| (a-5)g |
| x |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ��ѧʽ | CaCO3 | CaSO3 | CaC2O4 | Mg��OH��2 |
| Ksp | 4.96��10-9 | 4.96��10-9 | 2.34��10-9 | 5.61��10-12 |
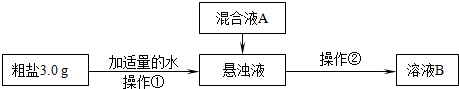
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
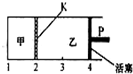 ��2011?������ģ����֪���淴Ӧ��A��s��+2B��g��?C��g��+D��g����H��0����һ���¶��½�1mol A��2molB������ͼ��ʾ�������У���2molC��2mol D�����������У���ʱ���ƻ���P��ʹ�ҵ��ݻ�Ϊ��2�����ں�����ʹ�������ڷ�Ӧ���ﵽƽ��״̬����ͼ��ʾ������K�����ƶ���������˵����ȷ���ǣ�������
��2011?������ģ����֪���淴Ӧ��A��s��+2B��g��?C��g��+D��g����H��0����һ���¶��½�1mol A��2molB������ͼ��ʾ�������У���2molC��2mol D�����������У���ʱ���ƻ���P��ʹ�ҵ��ݻ�Ϊ��2�����ں�����ʹ�������ڷ�Ӧ���ﵽƽ��״̬����ͼ��ʾ������K�����ƶ���������˵����ȷ���ǣ��������鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com