
��1����ʢ������ͭˮ��Һ���Թ�����백ˮ�������γ�������������Ӱ�ˮ���������ܽ�õ�����ɫ������Һ�����жԴ������˵����ȷ����
A����Ӧ����Һ�в������κγ��������Է�Ӧǰ��Cu2+��Ũ�Ȳ���
B�������ܽ����������ɫ���������[Cu��NH3��4] 2+
C����[Cu��NH3��4] 2+�У���Cu2+�����¶Ե��ӣ�NH3�ṩ�չ��
D����Ӧ�����Һ�м����Ҵ�����Һû�з����仯����Ϊ[Cu��NH3��4] 2+�������Ҵ�������Ӧ
��2��д����1�������е����ӷ���ʽ �� ��
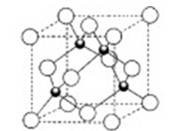
��3��CuԪ����ClԪ���γɵ�һ�����Ӿ���ľ����ṹ��ͼ��ʾ�����Ȼ���Ļ�ѧʽ�� �����û����ᄃ����ܶ�Ϊ�� g/cm3��NA��ʾ����٤����������þ���������� cm3��
��1��B ��1�֣�
��2��Cu2++2NH3��H2O=Cu(OH)2��+2NH4+ ��2�֣�
Cu(OH)2+4NH3=[Cu(NH3)4]2++2OH- ��2�֣�
��3��CuCl��1�֣� 398/��NA��2�֣�
����������1���γɵ���������������ͭ�������μӰ�ˮ��������ͭ�ܽ⣬��������ͭ���ӺͰ��������γ�����λ�������а��������壬ͨ���¶Ե��ӣ�ͭ����ͨ���չ�������ɵ��ӡ����ڸ���������Ҵ��е��ܽ��С�����Լ����Ҵ����γɾ��壬���ֻ��ѡ��B����ȷ�ģ�ǿ�ڶ��Ǵ���ģ���ѡB��
��2���йصķ���ʽΪCu2++2NH3��H2O=Cu(OH)2��+2NH4+��Cu(OH)2+4NH3=[Cu(NH3)4]2++2OH-��
��3�����ݾ����Ľṹ��֪������ͭԭ����8��1/8��6��1/2��4����ԭ��ȫ���ھ����ڣ�������4�������Ի�ѧʽΪCuCl�������ΪV����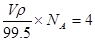 �����V��398/��NA��
�����V��398/��NA��


 ״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д�
״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1����ͼ��ʾΪ����ʯ����ѧʽΪNa3AlF6���ľ�����ͼ�С�λ�ڴ������嶥������ģ���λ�ڴ��������12������е��8��С����������ģ�����ͼ�С��е�һ�֣�ͼ�С�ֱ�ָ����������
��1����ͼ��ʾΪ����ʯ����ѧʽΪNa3AlF6���ľ�����ͼ�С�λ�ڴ������嶥������ģ���λ�ڴ��������12������е��8��С����������ģ�����ͼ�С��е�һ�֣�ͼ�С�ֱ�ָ����������| �۵�/K | �е�/K | ��״��ʱ��ˮ�е��ܽ�� | |
| H2S | 187 | 202 | 2.6 |
| H2O2 | 272 | 423 | ������Ȼ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
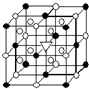 A����1����ͼ��ʾΪ����ʯ����ѧʽΪNa3AlF6���ľ�����ͼ�С�λ�ڴ������嶥������ģ���λ�ڴ��������12������е��8��С����������ģ���ͼ�С��е�һ�֣�ͼ�С�ֱ�ָ����������
A����1����ͼ��ʾΪ����ʯ����ѧʽΪNa3AlF6���ľ�����ͼ�С�λ�ڴ������嶥������ģ���λ�ڴ��������12������е��8��С����������ģ���ͼ�С��е�һ�֣�ͼ�С�ֱ�ָ����������| �۵�/K | �е�/K | ��״��ʱ��ˮ�е��ܽ�� | |
| H2S | 187 | 202 | 2.6 |
| H2O2 | 272 | 423 | ������Ȼ��� |
| 80m-135n |
| 18n |
| 80m-135n |
| 18n |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com