
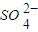 +2H2O
+2H2O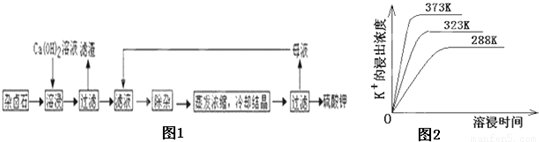
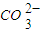 ?CaCO3��s��+
?CaCO3��s��+
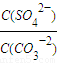 =
=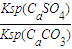 =
=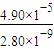 =1.75×104���ʴ�Ϊ��1.75×104��
=1.75×104���ʴ�Ϊ��1.75×104��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| SO | 2- 4 |
| CO | 2- 3 |
| SO | 2- 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������������һ�и߶���ѧ�����п������ƻ�ѧ�Ծ����������� ���ͣ������
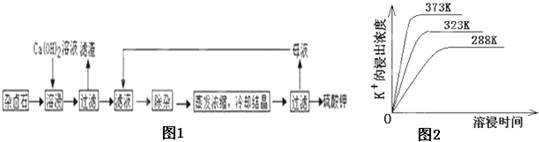
��13�֣���������±ʯ��K2SO4��MgSO4��2CaSO4��2H2O�����ڡ�������ˮ�д�������ƽ��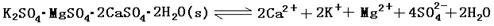
Ϊ�ܳ�����ü���Դ���ñ���Ca��OH��2��Һ�ܽ���±ʯ�Ʊ�����أ������������£�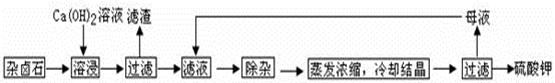
��1��������Ҫ�ɷ��� �� �Լ�δ����±ʯ��
��2���û�ѧƽ���ƶ�ԭ������Ca��OH��2��Һ���ܽ���±ʯ����K+��ԭ�� ��
��3�������ӡ������У��ȼ��� ��Һ��������Ȳ������ˣ��ټ��� ��Һ����ҺPH�����ԡ�
��4����ͬ�¶��£�K+�Ľ���Ũ�����ܽ�ʱ��Ĺ�ϵ��ͼ14����ͼ�ɵã������¶����ߣ� 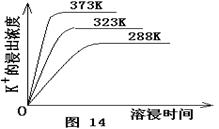
�� ����ͬʱ��K+�Ľ���Ũ�ȴ�
�� ����ͬʱ��K+�Ľ���Ũ�ȼ�С
�� ��Ӧ�����ʼӿ죬ƽ��ʱ�ܽ�ʱ��̡�
�� ��Ӧ���ʼ�����ƽ��ʱ�ܽ�ʱ��������
��5�������Կ�����̼����Ϊ�ܽ��������ܽ������лᷢ����
��֪298Kʱ�� Ksp(CaCO3)=2.80��10��9�� Ksp(CaSO4)=4.90��10��5 ������¶��¸÷�Ӧ��ƽ�ⳣ��K ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�������и߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��������±ʯ(K2SO4��MgSO4��2CaSO4��2H2O)���ڡ�������ˮ�д��������ܽ�ƽ�⣺
K2SO4��MgSO4��2CaSO4��2H2O(s) 2Ca2����2K����Mg2����4SO42-��2H2O��Ϊ�ܳ�����ü���Դ���ñ���Ca(OH)2��Һ�ܽ���±ʯ�Ʊ�����أ������������£�
2Ca2����2K����Mg2����4SO42-��2H2O��Ϊ�ܳ�����ü���Դ���ñ���Ca(OH)2��Һ�ܽ���±ʯ�Ʊ�����أ������������£�
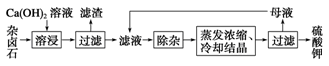
��1��������Ҫ�ɷ���________��CaSO4�Լ�δ����±ʯ��
��2���û�ѧƽ���ƶ�ԭ������Ca(OH)2��Һ���ܽ���±ʯ����K����ԭ��
��
��3�������ӡ������У��ȼ��� ��Һ��������Ȳ������ˣ��ټ��� ��Һ����ҺpH�����ԡ�
��4����ͬ�¶��£�K���Ľ���Ũ�����ܽ�ʱ��Ĺ�ϵ����ͼ����ͼ�ɵã������¶����ߣ�
�� ��
�� ��

���ܽ�����K����ƽ��Ũ������
��5�������Կ�����̼����Ϊ�ܽ��������ܽ������лᷢ����CaSO4(s)��CO32- CaCO3(s)��SO42-����֪298 Kʱ��Ksp(CaCO3)��2.80��10��9��Ksp(CaSO4)��4.90��10��5��������¶��¸÷�Ӧ��ƽ�ⳣ����K��
��
CaCO3(s)��SO42-����֪298 Kʱ��Ksp(CaCO3)��2.80��10��9��Ksp(CaSO4)��4.90��10��5��������¶��¸÷�Ӧ��ƽ�ⳣ����K��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�����к����ѧ�߶����ϣ����л�ѧ�Ծ��������棩 ���ͣ������
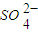 +2H2O
+2H2O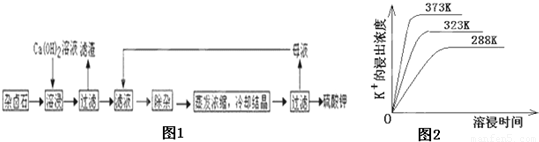
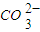 ?CaCO3��s��+
?CaCO3��s��+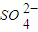
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com