
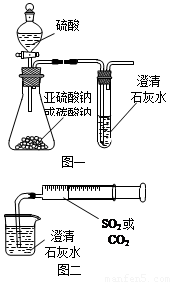
ijͬѧ����ͼһ��ʾ��װ����̽��CO2��SO2�����ʯ��ˮ�ķ�Ӧ�����ͨ��CO2���Կ����Ȼ��Ǻ���������ͨ��SO2û���ܿ�������������˼��������ͬѧ����ͼ����װ�ã��������ռ���ע�����������ؽ�����һ������һ�����ݵ�ͨ�����ʯ��ˮ�У����ܿ���ʯ��ˮ�ȱ�����ٳ����������ͨ��SO2�����������Ա�ͨ��CO2�졣
(1) ����ƿ��װ�����������ƣ�д����ƿ�ڷ�Ӧ�Ļ�ѧ����ʽ��
_______________________________________________________________��
(2)�Աȷ�������ʵ�飬����Ϊ��ͼһװ��ʵ��ʱ��ͨ��SO2���ܳ��ֻ��ǵ�ԭ������ǣ�__________________________________________________________��д����ʱ�Թ��ڷ�Ӧ�Ļ�ѧ����ʽ��______________________________________________________��
(3)��ͼ��װ��ʵ��ʱ������ͬ����ͨ��CO2��SO2��SO2�������ǡ�����������CO2���ԭ����_______________________________________________________________��
(4)��ͼһ����SO2��ʯ��ˮ��Ӧ��ʵ��ʱ��Ϊ��ֹSO2��Ⱦ������Ӧ��ʢ�й���Ũ�ռ���Һ����������SO2��д�������ڷ�����Ӧ�Ļ�ѧ����ʽ��
________________________________________________________________��

(1) Na2SO3��H2SO4�T�TNa2SO4��H2O��SO2?? (3��)
(2)���߷�Ӧʱֱ����������ʽ�ζ����������� (2��)
Ca(OH)2��2SO2�T�TCa(HSO3)2 (3��)
(3)SO2�ܽ�ȴ��Ҷ�Ӧ�����������Ա�̼��ǿ��ͨ���SO2��ʯ��ˮ��Ӧ�� (2��)
(4) SO2��2NaOH�T�TNa2SO3��H2O (3��)
�����ѣ�CO2��ʯ��ˮ��Ӧ���ɰ�ɫ����CaCO3��SO2Ҳ���ʯ��ˮ��Ӧ���ɰ�ɫ����CaSO3�����������ʱ��ɫ�������ܽ⣬���ԣ�һ����Ϊ������ʯ��ˮ������CO2��SO2��Ȼ����������Ľ����ǣ�ͨ��SO2û���ܿ�������������Ҫ��CO2��SO2���ʵ�������������ԭ������ˮ�е��ܽ�ȼ���Ӧ������Բ�ͬ����ֹSO2��Ⱦ������Ҫ����β������װ�ã��ռ����ʱ���ɵ���ΪNa2SO3�����ܿռ䣺����������Ӧ���������������ʱ������ʽ�Σ��������ʱ�������Ρ����ԣ���(2)�⻯ѧ����ʽд�ɣ�SO2��Ca(OH)2�T�TCaSO3??��H2O���÷֡���(4)�⻯ѧ����ʽд�ɣ�SO2��NaOH�T�TNaHSO3���÷֡�CO2��SO2�����ϵ����������㣺SO2�н�ǿ�Ļ�ԭ�ԣ�CO2û�У�SO2��Ư���ԣ�CO2û�С��������������뱾���أ���(3)����в�Ҫ�ἰ�����㡣
Ӧ�Բ��ԣ���������̽����ʵ���⣬̽����ʵ����ָ̽���о������δ֪���ʣ��˽���������������ɣ�����Щ���Ժͱ仯�������Լ�������������������ϵ�ȵ�һ��ʵ�顣������Ŀ�Ľ��Ҫ��ֹ˼ά���Ƶĸ��ţ�����ƽʱѧϰ��ij�����ʵ����ʲ���һ���о��ú�������̽����ʵ�������Ҫ��ʵ����ʵΪ���������ھ����ʵ����ʣ�����Ҫ���������Ϣ�����������������ȷ���


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧ����ͼһ��ʾ��װ����̽��CO2��SO2�����ʯ��ˮ�ķ�Ӧ�����ͨ��CO2���Կ����Ȼ��Ǻ���������ͨ��SO2û�п�������������˼����������ͬѧ����ͼ����װ�ã��������ռ���ע�����У������ؽ�����һ������һ�����ݵ�ͨ�����ʯ��ˮ�У�������ܿ���ʯ��ˮ�ȱ�����ٳ����������ͨ��SO2�������������Ա�ͨ��CO2�졣

��1������ƿ��װ�����������ƣ�д����ƿ�ڷ�Ӧ�Ļ�ѧ����ʽ��______________
______________________________��
��2���Աȷ�������ʵ�飬����Ϊ��ͼһװ��ʵ��ʱ��ͨ��SO2���ܳ��ֻ��ǵ�ԭ�������____________________________________��д����ʱ�Թ��ڷ�����Ӧ���ܻ�ѧ����ʽ��____________________________________��
��3����ͼ��װ��ʵ��ʱ������ͬ����ͨ��CO2��SO2��SO2���������ٱ����������CO2�죬��ԭ����___________________________________________��
��4����ͼһ����SO2��ʯ��ˮ��Ӧ��ʵ��ʱ��Ϊ��ֹSO2��Ⱦ������Ӧ��ʢ�й���Ũ�ռ���Һ����������SO2��д�������ڷ�����Ӧ�����ӷ���ʽ��____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���֣�ijͬѧ����ͼһ��ʾ��װ����̽��CO2��SO2�����ʯ��ˮ�ķ�Ӧ�����ͨ��CO2���Կ����Ȼ��Ǻ���������ͨ��SO2��������������˼�����ͬѧ����ͼ����װ�ã��������ռ���ע�����������ؽ�����һ������һ�����ݵ�ͨ�����ʯ��ˮ�У����ܿ���ʯ��ˮ�ȱ�����ٳ����������ͨ��SO2ʱ�����������Ա�ͨ��CO2�졣
���֣�ijͬѧ����ͼһ��ʾ��װ����̽��CO2��SO2�����ʯ��ˮ�ķ�Ӧ�����ͨ��CO2���Կ����Ȼ��Ǻ���������ͨ��SO2��������������˼�����ͬѧ����ͼ����װ�ã��������ռ���ע�����������ؽ�����һ������һ�����ݵ�ͨ�����ʯ��ˮ�У����ܿ���ʯ��ˮ�ȱ�����ٳ����������ͨ��SO2ʱ�����������Ա�ͨ��CO2�졣
��1���Աȷ�������ʵ�飬����Ϊ��ͼһװ��ʵ��ʱ��ͨ��SO2���ܳ��ֻ��ǵ�ԭ������ǣ�
��
��2����ͼ��װ��ʵ��ʱ������ͬ����ͨ��CO2��SO2��SO2�������ǡ�����������CO2���ԭ���� ��
��3����ͼһװ�ý���SO2��ʯ��ˮ��Ӧ��ʵ��������ȱ�ݣ���Ľ������ǣ�������Ϊ��װ��û��ȱ�ݣ��˿տ��Բ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��12�֣�ijͬѧ����ͼһ��ʾ��װ����̽��CO2��SO2�����ʯ��ˮ�ķ�Ӧ�����ͨ��CO2���Կ����Ȼ��Ǻ���������ͨ��SO2û���ܿ�������������˼��������ͬѧ����ͼ����װ�ã��������ռ���ע�����������ؽ�����һ������һ�����ݵ�ͨ�����ʯ��ˮ�У����ܿ���ʯ��ˮ�ȱ�����ٳ����������ͨ��SO2�����������Ա�ͨ��CO2�졣

��1���Աȷ�������ʵ�飬����Ϊ��ͼһװ��ʵ��
ʱ��ͨ��SO2���ܳ��ֻ��ǵ�ԭ������ǣ�
_________________��
��2����ͼ��װ��ʵ��ʱ������ͬ����ͨ��CO2��
SO2��SO2�������ǡ�����������CO2
���ԭ����______________________________________��
��3����ͼһ����SO2��ʯ��ˮ��Ӧ��ʵ��ʱ���Ӱ�ȫ�Ƕ�
����װ��Ӧ���θĽ���
_____________________________________________��
��4�������������ʵ������ʯ��ˮ�ȱ�����ٳ��塱���������ʯ��ˮ��Ũ���йء�Ϊ��̽��CO2ͨ�����ʯ��ˮ�е�ʵ��������������ݣ�
�� 20��ʱ��Ca(OH)2 ���ܽ��Ϊ��0.165g/100gˮ��
�� ��ͬŨ��ʯ��ˮ����CaCO3�������
| ����ʯ��ˮ��ˮ������� | 1:0 | 1:1 | 1:2 | 1:3 | 1:5 | 1:7 |
| ������CaCO3���������g/100ˮ�� | A | 0.110 | 0.073 | 0.055 | 0.037 | 0.028 |
�� �ϱ���A= g/100gˮ
�� ��1.01��105Pa CO2ѹ���£�CaCO3���ܽ��
| ����ѧ�¶�/K | 282 | 298 | 308 |
| CaCO3�ܽ�ȣ�g/100ˮ�� | 0.130 | 0.094 | 0.076 5 |
�� �ڲ�ͬѹǿ��CO2���£�CaCO3�ܽ�ȣ�18�棩
| P(CO2)/Pa | 0 | 1.40��104 | 9.95��104 |
| CaCO3�ܽ�ȣ�g/100ˮ�� | 0.001 3 | 0.023 3 | 0.108 6 |
��������������ݻش��������⣺
���ɱ���ͱ�����֪CaCO3�ܽ�ȵı仯�����ǣ�
�����������ݿ��Եó����ۣ����۲쵽��ʯ��ˮ�ȱ�����ٳ������������Ҫ��ʵ�������ǣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꼪��ʡ������ѧ�ڵڶ��������Ի�ѧ�� ���ͣ������
��12�֣�ijͬѧ����ͼһ��ʾ��װ����̽��CO2��SO2�����ʯ��ˮ�ķ�Ӧ�����ͨ��CO2���Կ����Ȼ��Ǻ���������ͨ��SO2û���ܿ�������������˼��������ͬѧ����ͼ����װ�ã��������ռ���ע�����������ؽ�����һ������һ�����ݵ�ͨ�����ʯ��ˮ�У����ܿ���ʯ��ˮ�ȱ�����ٳ����������ͨ��SO2�����������Ա�ͨ��CO2�졣
��1���Աȷ�������ʵ�飬����Ϊ��ͼһװ��ʵ��
ʱ��ͨ��SO2���ܳ��ֻ��ǵ�ԭ������ǣ�
_________________��
��2����ͼ��װ��ʵ��ʱ������ͬ����ͨ��CO2��
SO2��SO2�������ǡ�����������CO2
���ԭ����______________________________________��
��3����ͼһ����SO2��ʯ��ˮ��Ӧ��ʵ��ʱ���Ӱ�ȫ�Ƕ�
����װ��Ӧ���θĽ���
_____________________________________________��
��4�������������ʵ������ʯ��ˮ�ȱ�����ٳ��塱���������ʯ��ˮ��Ũ���йء�Ϊ��̽��CO2ͨ�����ʯ��ˮ�е�ʵ��������������ݣ�
�� 20��ʱ��Ca(OH)2 ���ܽ��Ϊ��0.165g/100gˮ��
�� ��ͬŨ��ʯ��ˮ����CaCO3�������
|
����ʯ��ˮ��ˮ������� |
1:0 |
1:1 |
1:2 |
1:3 |
1:5 |
1:7 |
|
������CaCO3���������g/100ˮ�� |
A |
0.110 |
0.073[��Դ:Zxxk.Com] |
0.055 |
0.037 |
0.028 |
�� �ϱ���A= g/100gˮ
�� ��1.01��105Pa CO2ѹ���£�CaCO3���ܽ��
|
����ѧ�¶�/K |
282 |
298 |
308 |
|
CaCO3�ܽ�ȣ�g/100ˮ��[��Դ:ѧ#��#��Z#X#X#K] |
0.130 |
0.094 |
0.076 5 |
�� �ڲ�ͬѹǿ��CO2���£�CaCO3�ܽ�ȣ�18�棩
|
P(CO2)/Pa |
0 |
1.40��104 |
9.95��104 |
|
CaCO3�ܽ�ȣ�g/100ˮ�� |
0.001 3 |
0.023 3 |
0.108 6 |
��������������ݻش��������⣺
���ɱ���ͱ�����֪CaCO3�ܽ�ȵı仯�����ǣ�
�����������ݿ��Եó����ۣ����۲쵽��ʯ��ˮ�ȱ�����ٳ������������Ҫ��ʵ�������ǣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com