
ijѧϰС��Ϊ�о��ͼ�̬��Ԫ�����ʵĻ�ԭ�ԣ���NH3Ϊ�����������ͼʵ�飨�г��豸��ȥ��

I�����������Ժ�رյ��ɼ�I���ɼ�II����ȼ�ƾ��ƣ�ͭ˿���Ⱥ��Һ©���Ļ�����A���������B��ʱ����ͨ���������Ӧһ��ʱ���Ϩ��ƾ��ơ�
II����D������������ʱ�رյ��ɼ�II�����ɼ�I
��1����֤���а��������������� �������ӵ���ʽΪ ��
��2��C��ͭ˿��Ϩ��Ƶƺ��Ա��ֺ���˵�����з�ӦΪ �ȷ�Ӧ������ȡ����ȡ�NH3��C�з�Ӧ�Ļ�ѧ����ʽΪ ��
��3��D�в�������ɫ����Ͱ��̣��������̵Ļ�ѧ����ʽΪ��
��4��F��Ҳ�����������̣�д���÷�Ӧ�з�Ӧ������ʵ���֮��n(NH3):n(Cl2)
��5���û�ѧ����ʽ����Ӧ����˵��A���ܲ�������NH3�����ԭ�� ���Ƚ�ijʱ��A�з�Һ©����Һƽ�ⳣ��K1������ƿ����Һ��ƽ�ⳣ��K2��С��ϵ��K1 K2(����<����=����>��)��
(6)װ����A���в��㣬Ӧ�������Ľ� ��
��1�������� A��ʪ��ĺ�ɫʯ����ֽ������ 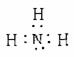 ���������������������� (2��)
���������������������� (2��)
��2�������� ���� ���� 4NH3 +5O2 ![]() ��4NO + 6H2O
��4NO + 6H2O
2NH3 +3CuO![]() 3Cu +N2+ 3H2O���������������������� ��3����
3Cu +N2+ 3H2O���������������������� ��3����
��3��HNO3 + NH3 = NH4NO3 ���� ks5u������������������������������������������������������������ ��2�֣�
��4��8:3���������������������������������������������������� ��1�֣�
��5��NH3 + H2O �� ![]() ������NH3.H2O����
������NH3.H2O����![]() ���� NH4+ + OH- ��H ��0��������ʹc(OH-)����ƽ�������ƶ���ͬʱ����������ˮ�ų������ȣ�Ҳʹƽ�������ƶ����������������� ��2�֣���
���� NH4+ + OH- ��H ��0��������ʹc(OH-)����ƽ�������ƶ���ͬʱ����������ˮ�ų������ȣ�Ҳʹƽ�������ƶ����������������� ��2�֣���
K1С��K2 �������� ��1�֣�
(6)װ����Aװ�÷�Һ©��������ƿ֮������һ��ѹƽ��ܡ����� ��1�֣�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����



| ||
| �� |
| ||
| �� |
| ||
| �� |
| ||
| �� |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�������ʡ�����ص�һ��ѧ������ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��13�֣�ijѧϰС��Ϊ�о��ͼ�̬��Ԫ�����ʵĻ�ԭ�ԣ���NH3Ϊ�����������ͼʵ�飨�г��豸��ȥ��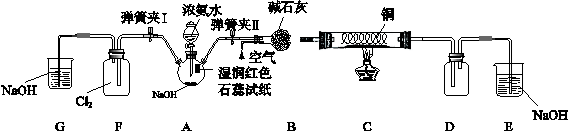
I�����������Ժ�رյ��ɼ�I���ɼ�II����ȼ�ƾ��ƣ�ͭ˿���Ⱥ��Һ©���Ļ�����A���������B��ʱ����ͨ���������Ӧһ��ʱ���Ϩ��ƾ��ơ�
II����D������������ʱ�رյ��ɼ�II�����ɼ�I
��1����֤���а��������������� �������ӵ���ʽΪ ��
��2��C��ͭ˿��Ϩ��Ƶƺ��Ա��ֺ���˵�����з�ӦΪ ��Ӧ������ȡ����ȡ���NH3��
C�з�Ӧ�Ļ�ѧ����ʽΪ ��
��3��D�в�������ɫ����Ͱ��̣��������̵Ļ�ѧ����ʽΪ��
��4��F��Ҳ�����������̣�д���÷�Ӧ�з�Ӧ������ʵ���֮��n(NH3):n(Cl2)
��5���û�ѧ����ʽ����Ӧ����˵��A���ܲ�������NH3�����ԭ�� ���Ƚ�ijʱ��A�з�Һ©����Һƽ�ⳣ��K1������ƿ����Һ��ƽ�ⳣ��K2��С��ϵ��K1 K2(�<����=����>��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�������ʡ������ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
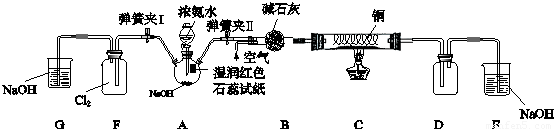
��13�֣�ijѧϰС��Ϊ�о��ͼ�̬��Ԫ�����ʵĻ�ԭ�ԣ���NH3Ϊ�����������ͼʵ�飨�г��豸��ȥ��
I�����������Ժ�رյ��ɼ�I���ɼ�II����ȼ�ƾ��ƣ�ͭ˿���Ⱥ��Һ©���Ļ�����A���������B��ʱ����ͨ���������Ӧһ��ʱ���Ϩ��ƾ��ơ�
II����D������������ʱ�رյ��ɼ�II�����ɼ�I
��1����֤���а��������������� �������ӵ���ʽΪ ��
��2��C��ͭ˿��Ϩ��Ƶƺ��Ա��ֺ���˵�����з�ӦΪ ��Ӧ������ȡ����ȡ���NH3��
C�з�Ӧ�Ļ�ѧ����ʽΪ ��
��3��D�в�������ɫ����Ͱ��̣��������̵Ļ�ѧ����ʽΪ��
��4��F��Ҳ�����������̣�д���÷�Ӧ�з�Ӧ������ʵ���֮��n(NH3):n(Cl2)
��5���û�ѧ����ʽ����Ӧ����˵��A���ܲ�������NH3�����ԭ�� ���Ƚ�ijʱ��A�з�Һ©����Һƽ�ⳣ��K1������ƿ����Һ��ƽ�ⳣ��K2��С��ϵ��K1 K2(�<����=����>��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�걱���д������߿���ѧһģ�Ծ��������棩 ���ͣ������
 3Cu+N2+3H2O
3Cu+N2+3H2O�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com