

���� ��1���ÿ����е�������Fe2+������Fe3+��Fe3+����ú�е�FeS2��FeΪ+2�ۣ���Ӧ���ɶ��������Ӻ���������ӣ��ݴ���д��
��2�����ݸƵ�ȼ����635kJ•mol-1�����ȼ����297kJ•mol-1������Ƶ������ʡ�H=-1434kJ•mol-1������
��Ca��s��+1/2O2��g���TCaO��s������H=-635kJ•mol-1��
��S��s��+O2��g���TSO2��g������H=-297kJ•mol-1��
��Ca��s��+S��s��+2O2��g���TCaSO4��s������H=-1434kJ•mol-1��
�ɸ�˹���ɢ�-��-�ټ���õ���
��3���ٸ�������Һ��n��SO32-����n��HSO3-��=10��1����HSO3-ռ���к����������ʵ�������Ϊ$\frac{1}{1+10}$=0.09�����ͼ�������
�ڢ��ݵ��װ��ͼ��Pt��I���缫Ϊ�����ӵõ�������������
���ݵ��װ��ͼ��Pt���缫Ϊ�������ʧ���������������
��4����������1.25��10-5mol��H2SO4���������ᷴӦ���ĵ�����������Һ���ٸ�������15.55mLNaOH��Һ������ú�����γɵ��������ĵ��������ƣ�������2NaOH+H2SO4=Na2SO4+2H2O������غ����ú���������������
��� �⣺��1���ÿ����е�������Fe2+������Fe3+����Ӧ�����ӷ���ʽΪ��4H++4Fe2++O2�T4Fe3++2H2O�����ɵ�Fe3+����ú�е�FeS2��FeΪ+2�ۣ���Ӧ���ɶ��������Ӻ���������ӣ����ӷ���ʽΪ��8H2O+FeS2+14Fe3+�T15Fe2++16H++2SO42-���ʴ�Ϊ��4H++4Fe2++O2�T4Fe3++2H2O��8H2O+FeS2+14Fe3+�T15Fe2++16H++2SO42-��
��2�����ݸƵ�ȼ����635kJ•mol-1�����ȼ����297kJ•mol-1������Ƶ������ʡ�H=-1434kJ•mol-1������
��Ca��s��+1/2O2��g���TCaO��s������H=-635kJ•mol-1��
��S��s��+O2��g���TSO2��g������H=-297kJ•mol-1��
��Ca��s��+S��s��+2O2��g���TCaSO4��s������H=-1434kJ•mol-1��
�ɸ�˹���ɢ�-��-�ٵ�CaO��s��+SO2��g��+1/2O2��g���TCaSO4��s������H=-1434kJ•mol-1+297kJ•mol-1+635kJ•mol-1=-502kJ•mol-1��
�ʴ�Ϊ��-502��
��3������Ϊ����Һ��n��SO32-����n��HSO3-��=10��1����HSO3-ռ���к����������ʵ�������Ϊ$\frac{1}{1+10}$=0.09�����ͼ��HSO3-ռ���к����������ʵ�������Ϊ0.09ʱ����Һ��pHԼΪ8.18���ʴ�Ϊ��8.18��
�ڢ��ɵ��װ��ͼ��Pt��I���缫Ϊ�����ӵõ���������������ӦʽΪ��2H++2e-�TH2�����ʴ�Ϊ��2H++2e-�TH2����
���ɵ��װ��ͼ��Pt���缫Ϊ�������ʧ��������������������������࣬�����ų���b%����Ũ�ȴ��ڼ����a%����Ũ�ȣ�
�ʴ�Ϊ��a%��b%��
��4������ú��1350�����ϵĿ����г��ȼ�գ�������Ԫ��ת��ΪSO2��������SO3��g������Ԫ��ת��ΪHCl��������������������˫��ˮ���գ�����Ԫ��ȫ��ת�������ᣬ����NaOH+HCl=NaCl+H2O��NaCl+Hg��OH��CN�THg��Cl��CN+NaOH����H2SO4�ζ�����1.25��10-5mol��H2SO4����2NaOH+H2SO4=Na2SO4+2H2O���������ᷴӦ���ĵ���������Ϊ1.25��10-5mol��2=2.5��10-5mol��������ú�����γɵ��������ĵ���������Ϊ0.1000��0.01555mol-2.5��10-5mol=0.00153mol����������غ�10.00g��ú�������Ϊ$\frac{0.00153mol}{2}$��$\frac{1000}{10}$��32=2.448g��
���Ը�ú�������������Ϊ$\frac{2.448g}{10.00g}$��100%=24.48%���ʴ�Ϊ��24.48%��
���� ���⿼���˹�ҵ����Ļ���ԭ�������鷽��ʽ����д����˹���ɵ�Ӧ�á����ص�ԭ���Լ��ζ������㣬�ۺ���ǿ���ѶȽϴ�ע���ͼ���л�ȡ��Ϣ��������ʵ����ʽ��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
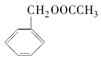 �������е�һ�֣������Դ���������ȡ��Ҳ��������ϩ�ͼױ�Ϊԭ�Ͻ����˹��ϳɣ�����һ�ֺϳ�·����ͼ��
�������е�һ�֣������Դ���������ȡ��Ҳ��������ϩ�ͼױ�Ϊԭ�Ͻ����˹��ϳɣ�����һ�ֺϳ�·����ͼ��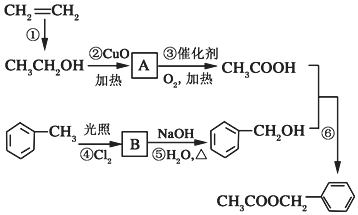
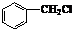 ��
��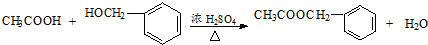 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ӵ��ȶ�������Ӽ��������Ĵ�С�� | |
| B�� | BF3��NCl3��H2O����������ԭ�Ӷ����������Ϊ8���ӽṹ����NCl3 | |
| C�� | NH4+��CH4���ڵȵ����壬���幹�Ͷ����������� | |
| D�� | ��[Cu��NH3��4]2+�����У�Cu2+�����¶Ե��ӣ�NH3�ṩ�չ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| t/min | n��CO��/mol | n��Cl2��/mol |
| 0 | 1.2 | 0.6 |
| 1 | 0.9 | |
| 2 | 0.2 | |
| 4 | 0.8 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
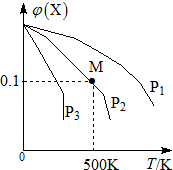 ij���淴ӦΪ2X��g��?3Y��g��+Z��g�������������X�����ʵ����������¶ȹ�ϵ��ͼ��ʾ�������ƶ���ȷ���ǣ�������
ij���淴ӦΪ2X��g��?3Y��g��+Z��g�������������X�����ʵ����������¶ȹ�ϵ��ͼ��ʾ�������ƶ���ȷ���ǣ�������| A�� | �ڸ�������M��Xƽ��ת����Ϊ$\frac{9}{11}$ | |
| B�� | ѹǿ��С��P3��P2��P1 | |
| C�� | ƽ�������Ч����ʹ������Է����������� | |
| D�� | �����¶ȣ��÷�Ӧƽ�ⳣ��K��С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ư���ڿ����в��ȶ���������Ư��ֽ�� | |
| B�� | Ư���е�CaCl2������е�CO2��Ӧ������CaCO3��Ư���ڿ����о��ñ��� | |
| C�� | NH3��ʹ��̪��Һ��죬���NH3�����������Ȫʵ�� | |
| D�� | ��ĥ��ʯ��ָ���벻�漰��ѧ��Ӧ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com