
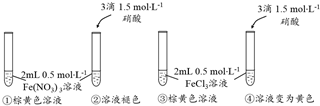
���� ��1����[Fe��H2O��6]3+��������ɫ��+nH2O?[Fe��H2O��6-n��OH��n]3-n����ɫ��+nH3O+��n=0��6����֪������HNO3��c��H+��������ƽ�������ƶ�����Һ�ɻ�ɫ��Ϊ��ɫ��
��2��ʵ��ڢܶԱȣ��Ȼ�����Һ�м������ᣬ��Һ��ɫ���䣬�Ȼ�����Һ��ɫΪ[FeCl4��H2O��2]-���£�
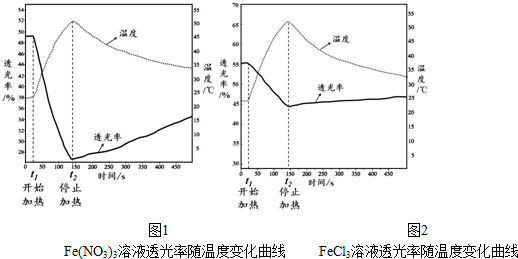
��3�������¶����ߣ�Fe��NO3��3��Һ������С��˵����Һ��[Fe��H2O��6-n��OH��n]3-nŨ������
��4�������¶���Һ��[FeCl4��H2O��2]-Ũ������
��5�����뺬�����������ʣ���ƽ���ƶ�������Һ��[FeCl4��H2O��2]-Ũ�ȣ�
��� �⣺��1��ʵ��٢ڶԱȣ���������Һ����������Һ����Һ��������Ũ��������Һ��ɫ��ȥ����Ϣ����ƽ�������ƶ���
�ʴ�Ϊ������HNO3��c��H+�������´�ƽ�������ƶ�����Һ�ɻ�ɫ��Ϊ��ɫ��
��2���Թܢڡ����м��������HNO3������Һ��ɫ����������Һ�Գʻ�ɫ��˵���Ȼ�����Һ��ɫΪ[FeCl4��H2O��2]-���£�
�ʴ�Ϊ���Թܢڡ����м��������HNO3������Һ��ɫ����������Һ�Գʻ�ɫ��
��3���¶����ߣ�����ƽ��[Fe��H2O��6]3++nH2O?[Fe��H2O��6-n��OH��n]3-n+nH3O+�����ƶ���[Fe��H2O��6-n��OH��n]3-nŨ��������Һ��ɫ���
�ʴ�Ϊ�����
��4��������ͬ�¶�ʱ��FeCl3��Һ�������¶ȱ仯�������Դ���Fe��NO3��3��Һ��˵����FeCl3��Һ�д���ˮ�������ӵ�ˮ��ƽ��֮�⣬������[FeCl4��H2O��2]-+
4H2O?[Fe��H2O��6]3++4Cl-��
�ʴ�Ϊ��������ͬ�¶�ʱ��FeCl3��Һ�������¶ȱ仯�������Դ���Fe��NO3��3��Һ��˵����FeCl3��Һ�д���ˮ�������ӵ�ˮ��ƽ��֮�⣬������[FeCl4��H2O��2]-+
4H2O?[Fe��H2O��6]3++4Cl-����H��0��
��5������ʵ��ڢܿ�֪����ڵ���Һ�м��뺬�����������ʣ�������Һ��[FeCl4��H2O��2]-Ũ�ȣ�����֤��ʵ�鷽����֤��4���н��ۣ�
�ʴ�Ϊ���ȵμ�HNO3���ٵμӼ���NaCl��Һ���������Һ�������¶ȸı�ı仯�����
���� ���⿼���˶�̽��ʵ�鷽���������ۡ���Ϣ��ȡ��Ǩ�����ã�ע�������ö���ʵ����з������Ƕ���ʵ�ۺ������Ŀ��飬�ѶȽϴ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
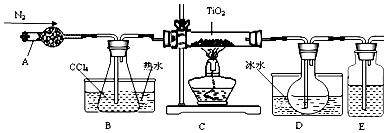
| ���� | �۵�/�� | �е�/�� | ���� |
| CCl4 | -23 | 76 | ��TiCl4���� |
| TiCl4 | -25 | 136 | ����ʪ������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
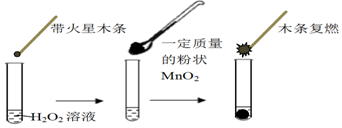
| MnO2������/g | 0.1 | 0.2 | 0.4 |
| 40sĩO2���/mL | 49 | 61 | 86 |
| �Թ� | �� | �� | �� |
| �μ��Լ� | 5��0.1mol•L-1FeCl3 | 5��0.1mol•L-1 CuCl2 | 5��0.3mol•L-1 NaCl |
| ������ ����� | �Ͽ����ϸС���� | ��������ϸС���� | �����ݲ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
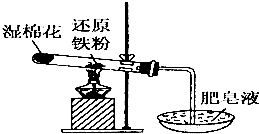 ������û����������ʱ������ˮ��������Ӧ���������£�����ˮ�����ܷ�Ӧ��С���������ʵ��̽��������ˮ������Ӧ���������
������û����������ʱ������ˮ��������Ӧ���������£�����ˮ�����ܷ�Ӧ��С���������ʵ��̽��������ˮ������Ӧ����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ʵ�鲽�� | Ԥ������ |
| ����һ���ý�ͷ�ι�ȡ��A�Թ��е���Һ����ˮϡ�ͺ���װ���Թܢ��б��� | |
| �����������Fe3+�����Թܢ��У����뼸��KSCN��Һ | ��Һ����Ѫ��ɫ |
| ������������Fe2+�����Թܢ��У���������KMnO4��Һ�����ȵ��뼸��ϡ���ᣩ | �Ϻ�ɫ��dz������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
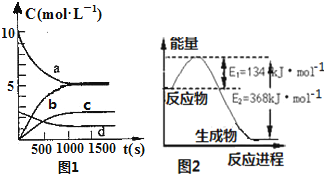 ���ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���ã����ٵ����������ڴ����е��ŷ��ǻ�����������Ҫ����֮һ��
���ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���ã����ٵ����������ڴ����е��ŷ��ǻ�����������Ҫ����֮һ��| t��s�� | 0 | 500 | 1000 | 1500 |
| n��NO2����mol�� | 20 | 13.96 | 10.08 | 10.08 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
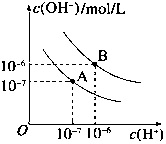 ��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ��
��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com