
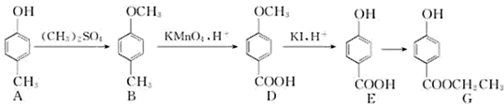
| ���� |
| �� |
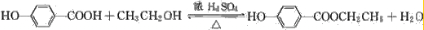
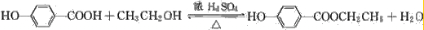
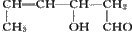
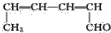
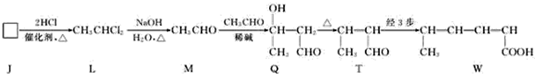
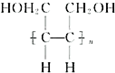
| ���� |
| �� |
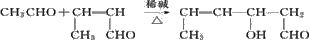
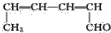
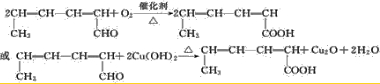
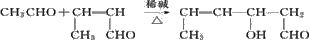



 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| 690 |
| a |
| 690 |
| a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�갲��ʡ������ʮУ�������������ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
����������һ�r������ʳƷ���Ӽ���ʹ��ʱ�����ϸ������������Ϊ���ijʳƷ���������κ�����ͨ����1kg��Ʒ�к�NaNO2��������)��ij�о�С���������������ʵ�鷽����
(1)��ɫ����B�������Ժ���ɫ��B�Ļ�ѧʽΪ_______д���������з�Ӧ�����ӷ���ʽ_______
(2)��ɲ���ƽ�ҷ������з�Ӧ�����ӷ���ʽ
MnO4-+ NO2-+ = Mn2++ NO3-+ ,
(3)�ҷ�������������100mL0.0010mol/L(NH4)2Fe(SO4)2����Һ������ȷ������Ʒ����������Ҫ�������У���Ͳ���ձ���_______������Һʱ�����ݵIJ���������______
(4)��ȡ��Ʒag�����ҷ������вⶨ��ȷ��ȡ12.00mL0.0005mol/L�����Ը������ ��Һ����ͯ������ҺA��Ӧ����Ӧ����Һ��0.0010mol/L(NH4)2Fe(SO4)2����Һ�ζ�����ɫ ��Һ�պ���ȥ���ظ�����ʵ��2�Σ�ƽ������(NH4)2Fe(SO4)2��Һ10.00mL.��1kg��Ʒ�� NaNO2������Ϊ_______mg.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.�����ϸ�����������ĵ⡢п��Ԫ�ص�������
B.���ࡢ��֬�������ʡ�ά���ء����κ�ˮ������Ҫ����ҪӪ����
C.����ҩ���ֶ��������밴ҽ����ҩ��˵����ʹ��ҩ�����ҩ�ﲻ����Ӧ
D.��ɫ������ζ������������ʳƷ���Ӽ����ɸ���ʳƷ��ɫ��ζ����������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com