
���л��ﻯѧ������
ƻ����㷺������ƻ����ˮ���Ĺ����У���һ�ֳ��õ�ʳƷ���Ӽ������ⶨ��ƻ�������Է�������Ϊ134��������Ԫ�ص���������Ϊ��w(c)="35.82%" W(H)=4.86%,����Ϊ�������д���5�ֲ�ͬ��ѧ������Hԭ�ӡ�1molƻ��������2molNaHCO3��ȫ��Ӧ������������Na��Ӧ����1.5molH2�ġ�����ϩΪԭ���˹��ϳ�ƻ�������·���£�

��֪��
��ش��������⣺
��1��ƻ����ķ���ʽΪ_______��A���ʵ�����Ϊ_______��
��2��F�к��еĹ�����������_______��G+B��H�ķ�Ӧ������_______��
��3���ںϳ���·�У�C��D��һ���跴Ӧ��Ŀ����_____��
��4��D��E��Ӧ�Ļ�ѧ����ʽΪ_________��
��5��ƻ�����NaHCO3��ȫ��Ӧ�Ļ�ѧ����ʽΪ________��
��6����ƻ���Ậ����ͬ����������Ĺ����ŵ�ͬ���칹��Ľṹ��ʽΪ____��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��4�֣�ij�л������Է�������Ϊ102�������л��ﺬ̼���⡢������Ԫ�أ����к������������Ϊ9.8%����������ԭ�Ӹ���Ϊ��ԭ�ӵ�5�������л���ķ���ʽ�� �������л�����������Ʒ�����Ӧ����ȡ5.1 g ���л��������Na��Ӧ������ܲ�����̬������ L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(14��)ij��A���л���ѧ��ҵ�Ļ���ԭ�ϣ������������������һ�����ҵ�ʯ�ͻ�����չˮƽ��A����һ��ֲ���������ڼ���A�ɷ�����ͼ��ʾ��һϵ�л�ѧ��Ӧ��
��ͼ�ش��������⣺
(1)д��A��C��D�Ľṹ��ʽ��A________�� C________�� D________��
(2)д���٢�������Ӧ�Ļ�ѧ����ʽ����ע����Ӧ���ͣ�
��________________________________________________��Ӧ����Ϊ ____________��
��_______________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ѧѡ��-�л���ѧ��������15�֣���ϩ��Ϊԭ�ϣ��ϳ�ijЩ�߾����·�����£�
��֪�� ����д��
�����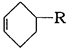 ��
��
��CH3CH��CHCH3��������____________����X�к��еĹ�������______________��
��A��B�Ļ�ѧ����ʽ��_____________����D��E�ķ�Ӧ������_______________��
�ɼ�Ϊ����F����NaHCO3��Ӧ����CO2��
�������й�˵����ȷ����_______________��
a���л���Z�ܷ���������Ӧ b���л���Y��HOCH2CH2OH��Ϊͬϵ��
c���л���Y�ķе��B�� d���л���F���뼺�������۳ɾۺ���
��Y��ͬ���칹���ж��֣�д�����ӽṹ�к�������������ͬ���칹���������__ ___��
��Z��W�Ļ�ѧ����ʽ��__________________��
�ʸ߾���H�Ľṹ��ʽ��_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������F�Ǻϳ�ij�ֿ���ҩ����м��壬��ϳ�·�����£�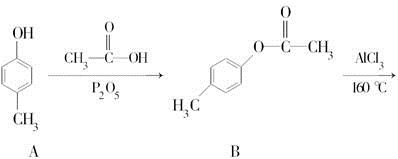



(1)������F�еĺ������������ʻ��� �� (�����������)��
(2)�ɻ�����D���ɻ�����E�ķ�Ӧ������ ��
(3)�ɻ�����A���ɻ�����B�Ļ�ѧ����ʽ�� ��
(4)д��ͬʱ��������������D��һ��ͬ���칹��Ľṹ��ʽ�� ��
�ٷ�������4�ֲ�ͬ��ѧ��������ԭ�ӣ����ܷ���������Ӧ������һ��ˮ�����Ҳ�ܷ���������Ӧ���۷����б�������ԭ�Ӳ�ֱ��������
(5)����������( )��һ����Ҫ�Ļ���ԭ�ϡ���д���Ա�����ȩ���Ҵ�Ϊ��Ҫԭ���Ʊ������������ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�
)��һ����Ҫ�Ļ���ԭ�ϡ���д���Ա�����ȩ���Ҵ�Ϊ��Ҫԭ���Ʊ������������ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪����A����ʽΪC3H4O2�������ԡ�FΪ���߸�ԭ����ɵĻ�״�ṹ������ʽΪC6H8O4 ����������¿�ͼ�ش�����
��1��A�Ľṹ��ʽΪ
��2����Ӧ�ٵķ�Ӧ����Ϊ
��3��������B�к��������ŵ�������
��4��D��E����F�Ļ�ѧ����ʽ
D��E����1:1��ӦҲ�����ɸ߾����д�����ɸø߾���Ļ�ѧ��Ӧ����ʽ��
��5��G����H�Ļ�ѧ����ʽ
��6��д��C��ͬ���칹���������������ʵĽṹ��ʽ �� �� ������д3����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������������C3H6�ϳ��л��߷���E����C6H14������ͼ����ش��������⣺ (1)�١���������ȡ����Ӧ���� ��
(1)�١���������ȡ����Ӧ���� ��
(2)C6H14�ĺ˴Ź�������ֻ�����ַ壬��C6H14�Ľṹ��ʽΪ ��д��E�Ľṹ��ʽ�� ��
(3)д��B����Cu(OH)2��Ӧ�Ļ�ѧ����ʽ�� ��
(4)D��ͬ���칹��ܶ࣬��������������ͬ���칹���� �֣�������ԭ�Ӻ˴Ź������������ٵĽṹ��ʽΪ ��
�ٺ�̼̼˫��������ˮ�⡡���ܷ���������Ӧ
(5)��������ѧ֪ʶ����ͼ�������Ϣ�����Ҵ�Ϊ��Ҫԭ��ͨ���������ܺϳɻ�����(���Լ���ѡ)��д����һ���͵�������ѧ��Ӧ�Ļ�ѧ����ʽ(�л�����д�ṹ��ʽ)�� �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Լ�����������( )�����ںϳ�ҩ����м��塣���������ת����ϵ�ش��й�����
)�����ںϳ�ҩ����м��塣���������ת����ϵ�ش��й�����
(1)D�к��й����ŵ�������________��A��E�ķ�Ӧ����Ϊ______��
(2)G�Ľṹ��ʽΪ_______��
(3)д��1�����������ұ�����ֻ��һ��ȡ������C8H8O2��ͬ���칹�� ��
(4)�����( )�ǶԼ�������������ͬ���칹�壬�������������ܷ�����Ӧ����________(�����)��
)�ǶԼ�������������ͬ���칹�壬�������������ܷ�����Ӧ����________(�����)��
| A��NaOH��Һ | B��NaHCO3��Һ | C��KMnO4/H+ | D��FeCl3��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������̼�����л��ϳ��зdz���Ҫ�ķ�Ӧ�����磺
��Ӧ��
�� ͨ������·�߿ɺϳ�(��)��
ͨ������·�߿ɺϳ�(��)��
��1�����ķ���ʽΪ ��1mol��������ȫȼ����Ҫ���� mol O2.��
��2������)����������NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ ��
���Ľṹ��ʽΪ �� �����ɣ���ʱ�����ܵõ���һ�ָ�����C6H8O4���÷�Ӧ�Ļ�ѧ����ʽΪ ���÷�Ӧ�ķ�Ӧ������ ��
�����ɣ���ʱ�����ܵõ���һ�ָ�����C6H8O4���÷�Ӧ�Ļ�ѧ����ʽΪ ���÷�Ӧ�ķ�Ӧ������ ��
��4���Զ��ȱ� Ҳ�����л���(��) (����)�������Ʒ�Ӧ�ٵ�ϵ�з�Ӧ���������л���Ľṹ��ʽΪ ��
Ҳ�����л���(��) (����)�������Ʒ�Ӧ�ٵ�ϵ�з�Ӧ���������л���Ľṹ��ʽΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com