
| 102��9.8% |
| 1 |
| 10 |
| 2 |
| 102-10-16��2 |
| 12 |
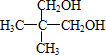 ����ӦΪ
����ӦΪ ��
��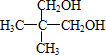 ����AΪ
����AΪ ���ݴ˽��
���ݴ˽��| 102��9.8% |
| 1 |
| 10 |
| 2 |
| 102-10-16��2 |
| 12 |
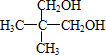 ����ӦΪ
����ӦΪ ��
��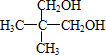 ����AΪ
����AΪ ��
��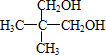 ��
�� ��
�� ������ͬ�����š�������֧����ͬ���칹��Ľṹ��ʽ�У�
������ͬ�����š�������֧����ͬ���칹��Ľṹ��ʽ�У�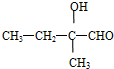 ��
��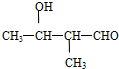 �ȣ�
�ȣ� ��
�� ��
�� ��������һ������ͬ���칹�壬���칹��������������ˮ�⣬����������Է���������ͬ�Ļ������Ϊ���������������һ�ֵķ�������2��������Ϊ2-������A��ͬ���칹��ΪCH3COOCH��CH3��2���˷�Ӧ�Ļ�ѧ����ʽ��CH3COOCH��CH3��2+H2O
��������һ������ͬ���칹�壬���칹��������������ˮ�⣬����������Է���������ͬ�Ļ������Ϊ���������������һ�ֵķ�������2��������Ϊ2-������A��ͬ���칹��ΪCH3COOCH��CH3��2���˷�Ӧ�Ļ�ѧ����ʽ��CH3COOCH��CH3��2+H2O | ���� |
| �� |
| ���� |
| �� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��3.4 g |
| B��0.34 g |
| C��1.36 g |
| D��4.48 g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��FeBr2��Cl2 |
| B��C6H5ONa��CO2 |
| C��HCl��Na2CO3 |
| D��Ca��HCO3��2��NaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��
�� ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 850�� |
| A��1��1 | B��1��5 |
| C��9��1 | D��1��10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ��ʵ���������Լ���ǩ�ϵIJ������ݣ��ݴ��ж�
��ͼ��ʵ���������Լ���ǩ�ϵIJ������ݣ��ݴ��ж��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com