
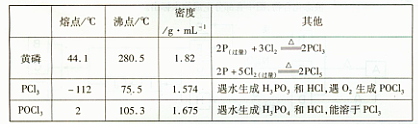
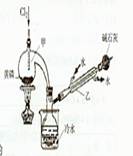
 5.00mL���������c1mol��L-lV1mL����Һ����ַ�Ӧ������c2mol��L-1Na2S2O3��Һ�ζ������ĵ⣬�յ�ʱ����V2mLNa2S2O3��Һ��
5.00mL���������c1mol��L-lV1mL����Һ����ַ�Ӧ������c2mol��L-1Na2S2O3��Һ�ζ������ĵ⣬�յ�ʱ����V2mLNa2S2O3��Һ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 2CrO42��+2H+���ƣ����µζ��յ��ͺ�
2CrO42��+2H+���ƣ����µζ��յ��ͺ�| ʵ����� | FeSO4��Һ���������/mL | |
| �ζ�ǰ | �ζ��� | |
| 1 | 0.10 | 16.20 |
| 2 | 0.30 | 15.31 |
| 3 | 0.20 | 15.19 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������������������ȡ������������ | B���÷�Һ©�����뻷�����ˮ�Ļ��Һ�� |
| C����Ũ��ˮϴ������������Ӧ���Թ� | D���ڱ�����ˮ�Ļ�����м���������ȡ�屽 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ֻ�Т٢� | B��ֻ�Тۢܢ� | C��ֻ�Т٢ڢۢ� | D���٢ڢۢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ʽ�ζ�����װҺǰδ�ñ�����Һ��ϴ2��3�� |
| B����ʼʵ��ʱ��ʽ�ζ��ܼ��첿�������ݣ��ڵζ�������������ʧ |
| C���ζ�ǰƽ�Ӷ������ζ����Ӷ��� |
| D��ʢNaOH��Һ����ƿ�ζ�ǰ��NaOH��Һ��ϴ2��3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 NaCl���ʣ�Ϊ��
NaCl���ʣ�Ϊ�� ����Ʒ�д����������������ͬѧ����ͼװ�ü��Լ�����ʵ�飨�г������ԣ�����д���пհ״���
����Ʒ�д����������������ͬѧ����ͼװ�ü��Լ�����ʵ�飨�г������ԣ�����д���пհ״���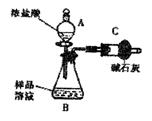
 ���ظ�ʹ�ã��г������ԣ�
���ظ�ʹ�ã��г������ԣ�
ѡ�õ� ���� ���� | | | | | |
| ���ӵ�ҩƷ����Ҫ�IJ��� | | | | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| �ζ����� | ���������mL�� | NaOH��Һ���������mL�� | |
| �ζ�ǰ | �ζ��� | ||
| 1 | 20.00 | 0.00 | 18.10 |
| 2 | 20.00 | 0.00 | 16.30 |
| 3 | 20.00 | 0.00 | 16.22 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
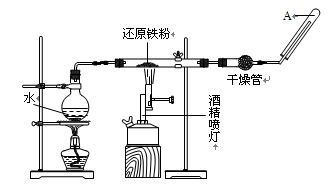
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com