
�ϳɰ���ҵ�Թ���������Ҫ���壬�������ᡢ�ϳ���ά�Լ�Ⱦ�ϵ�
��1����֪ijЩ��ѧ���ļ����������±���
|
��ѧ�� |
N��N |
H��H |
N��H |
|
����kJ��mol��1 |
946 |
436 |
390 |
�ϳɰ����Ȼ�ѧ��Ӧ����ʽΪ ��
��2�������£���NaCl�뱥�Ͱ�ˮ�Ļ����Һ��ͨ�����CO2����NaHCO3�����������÷�Ӧ�Ļ�ѧ����ʽΪ ��������Һ�����ӵĵ���غ�ʽ�� ������NaCl�뱥��CO2�Ļ����Һ��ͨ�백������û��NaHCO3����������ԭ���� ��
��3��������ˮ(��NH3��NaOH��Na2SO4)�����ŷŻ����ˮ�帻Ӫ��������ͼͨ��ֱ�ӵ绯ѧ��������Ч��ȥij�������������������ӵ���������Ϊ �����a��������b���������������У�b������pH �������С�����䡱����������Ӧ����ʽΪ ��
��1��N2(g)+3H2(g) 2NH3(g)
��H=-86kJ/mol
2NH3(g)
��H=-86kJ/mol
��2��NaCl+CO2+NH3��H2O=NH4Cl+NaHCO3��
c��Na+��+c(H+)+c��NH4+)=c(Cl-)+c��HCO3-)+2c��CO32-)+c��OH-)
������̼���ܽ��С�����ɵ�̼�����̫�٣���û��̼����������
��3����a�� ���� 2NH3-6e-+6OH-=N2+6H2O
��������
���������(1)�ɼ��������ȣ�946+3��436=2254kJ���½��ϳɷ��ȣ�6��390=2340kJ�����ȶ࣬��ѧ��Ӧ����
Ϊ���ȣ��ų�����86kJ������д������Ȼ�ѧ����ʽ��ͬʱע�⣺�������ʵľۼ�״̬��‚������λ��
kJ��mol��1��(2)���ݷ�Ӧ��Ͳ�������غ㶨�ɣ�����д����ط���ʽ��������Һ�ʵ����ԣ��������
�����ڸ����������д������غ㣻������̼��ˮ�е��ܽ�Ⱥ�С�����ɵ�̼���������Ũ�Ⱥ�С��
��û��̼��������������3���������������������������ӷŵ磬ʣ�����������ӣ���ҺpH����
���㣺���鷴Ӧ�ȵļ��㣬��⻯ѧ����ʽ��д��


 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д� Сѧ��ѧ������ѿڶ���ϵ�д�
Сѧ��ѧ������ѿڶ���ϵ�д� ������Ӧ�������������ϵ�д�
������Ӧ�������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �ϳɰ���ҵ�Ի�ѧ������ҵ������Ҫ���壮
�ϳɰ���ҵ�Ի�ѧ������ҵ������Ҫ���壮
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
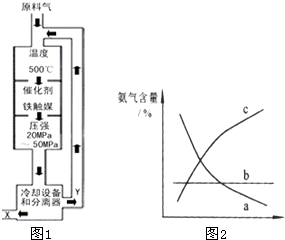 ��2008?��ɽһģ����1���ϳɰ���ҵ�Ի�ѧ��ҵ������ҵ������Ҫ���壮��ҵ�ϳ�
��2008?��ɽһģ����1���ϳɰ���ҵ�Ի�ѧ��ҵ������ҵ������Ҫ���壮��ҵ�ϳ�| 20-50MPa | 500�桢����ú |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��ѧ�� | N��N | H-H | N-H |
| ����kJ?mol-1 | 946 | 436 | 390 |

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com