
����һ������������һ�������£���������ת�������Ƶ�ˮ����ͱ��ӡ�
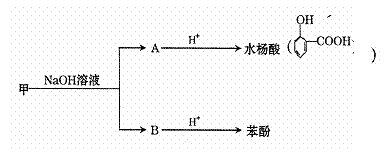
��ش��������⣺
��1��ˮ����ķ���ʽ�� ��
��2�����к��������ŵ������� ��
��3��������������ȡ���������������ˮ�����ͬ���칹���� �֡�
��4��д����������������Һ�з�����Ӧ�Ļ�ѧ����ʽ
��
��5���û�ѧ����ʽ˵��ˮ��������Աȱ��ӵ�����ǿ��ҩƷ����ѡ����
��
��6��ˮ���������б仯

���ҵĽṹ��ʽ�� ��
 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�����ֶ���2007��߿��л���ѧԤ���� ���ͣ�022
| |||||||||||||||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭������һ�и߶���ѧ�����п��Ի�ѧ�Ծ���ѡ�ޣ��������棩 ���ͣ������
ij��Ӧ�з�Ӧ�����������У�FeCl2��FeCl3��CuCl2��Cu��
��1����������Ӧ��Ƴɵ�ԭ�����ͼ����ʾ����ش��������⣺


��ͼ��X��Һ��_____________________________��
��ʯī�缫�Ϸ����ĵ缫��ӦʽΪ__________________________________________��
��ԭ��ع���ʱ�������е�____________(�K������Cl����)���Ͻ���X��Һ�С�
��2����������Ӧ��Ƴɵĵ�����ͼ����ʾ�����ձ��н��������ӵ����ʵ��������ת�Ƶ����ʵ����ı仯��ϵ��ͼ������ش��������⣺
��M��__________����
��ͼ���еĢ�����______________�ı仯��
�۵�����ת��Ϊ2 molʱ�������ձ��м���________ L 5 mol��L��1 NaOH��Һ����ʹ���еĽ��������ӳ�����ȫ��
��3��������Ҫ�������������(Na2FeO4)��һ����������ˮ�����������кܶ��ŵ㡣
�ٸ���������������֮һ�ǵ�ⷨ����ԭ��ΪFe��2NaOH��2H2O =Na2FeO4��3H2��������ʱ�����ĵ缫��Ӧʽ��__________________________________��
�ڸ���������������֮������ǿ���Խ�������NaClO����Fe(OH)3���ɸ������ơ��Ȼ��ƺ�ˮ���÷�Ӧ�����ӷ���ʽΪ_______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һ������������һ�������£���������ת�������Ƶ�ˮ����ͱ��ӡ�
 ��ش��������⣺
��ش��������⣺
��1��ˮ����ķ���ʽ�� ��
��2�����к��������ŵ������� ��
��3��������������ȡ���������������ˮ�����ͬ���칹���� �֡�
��4��д����������������Һ�з�����Ӧ�Ļ�ѧ����ʽ
��
��5���û�ѧ����ʽ˵��ˮ��������Աȱ��ӵ�����ǿ��ҩƷ����ѡ����
��
��6��ˮ���������б仯
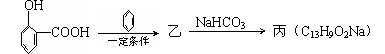
���ҵĽṹ��ʽ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com