
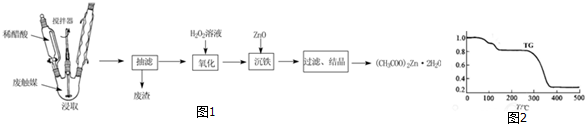
���� �ϴ�ý�ô����ȡ��ZnO��FeO����ᷴӦ�ܽ⣬��CH3COO��2ZnҲ�ܽ⣬����̿����Ӧ�����˳�ȥ����̿�Ȳ��������ʣ�����������⽫Fe2+����ΪFe3+���ټ���ZnO������ҺpH��ʹFe3+ת��Ϊ�����������������˳�ȥ����Һ�ᾧ�õ���CH3COO��2Zn��
��1�����Ȳ�ͬʱ����������������߽�ȡ���ʺͽ�ȡ�ʣ�
��2����������̼��Ӧ���������ڻ���̿���ڵĴ���пȫ���ͷų���������ƿ������©����
��3��˫��ˮ���������ԣ��ܹ���Fe2+����ΪFe3+��
��4����CH3COO��2Zn��370��C��ȫ�ֽ�ΪZnOͬʱ����CO2��һ�ֺ�����������������غ�֪������������Ļ�ѧʽΪC3H6O����Ϻ˴Ź�������ֻ��1��壬ֻ���DZ�ͪ��CH3COCH3����
��� �⣺�ϴ�ý�ô����ȡ��ZnO��FeO����ᷴӦ�ܽ⣬��CH3COO��2ZnҲ�ܽ⣬����̿����Ӧ�����˳�ȥ����̿�Ȳ��������ʣ�����������⽫Fe2+����ΪFe3+���ټ���ZnO������ҺpH��ʹFe3+ת��Ϊ�����������������˳�ȥ����Һ�ᾧ�õ���CH3COO��2Zn��
��1�����Ȳ�ͬʱ������������Ŀ�ģ���߽�ȡ���ʺͽ�ȡ�ʣ�
�ʴ�Ϊ����߽�ȡ���ʺͽ�ȡ�ʣ�
��2����������̼��Ӧ���������ڻ���̿���ڵĴ���пȫ���ͷų����������ʻ�����ߣ�����ʱ�õĹ������������У�����ƿ������©����
�ʴ�Ϊ���������ڻ���̿���ڵĴ���пȫ���ͷų���������ƿ������©����
��3��˫��ˮ���������ԣ��ܹ���Fe2+����ΪFe3+���ټ���ZnO������ҺpH��ʹFe3+ת��Ϊ�����������������˳�ȥ��
�ʴ�Ϊ����Fe2+����ΪFe3+��
��4����CH3COO��2Zn��370��C��ȫ�ֽ�ΪZnOͬʱ����CO2��һ�ֺ�����������������غ�֪������������Ļ�ѧʽΪC3H6O����Ϻ˴Ź�������ֻ��1��壬ֻ���DZ�ͪ��CH3COCH3������Ӧ����ʽΪ��CH3COO��2Zn$\frac{\underline{\;\;��\;\;}}{\;}$CH3COCH3+CO2��+ZnO��
�ʴ�Ϊ��CH3COO��2Zn$\frac{\underline{\;\;��\;\;}}{\;}$CH3COCH3+CO2��+ZnO��
���� ���⿼����ʵ�黯ѧ�е����ʵ��Ʊ������֪ʶ���ؼ��ǶԹ������̵����⣬��2���г��˲ٲ�������Ϊ�״��㣬��ѧ�������漰��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
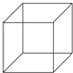 ������Ľṹ��ʽ��ͼ��ʾ��ÿ��������һ��̼ԭ�ӣ���
������Ľṹ��ʽ��ͼ��ʾ��ÿ��������һ��̼ԭ�ӣ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������һ����NO2��CO | B�� | ���������һ��û��H2��NH3 | ||
| C�� | ����ȷ������������Ƿ���NH3 | D�� | ���������������HCl���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
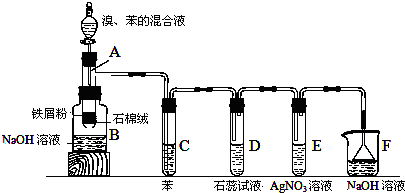
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ũ��ˮ�еμ�FeCl3������Һ���Ƶ�Fe��OH��3���� | |
| B�� | CH3COONa��Һ�еμ�Ũ�����c��CH3COO-������ | |
| C�� | Ca��HCO3��2��Һ�����NaOH��Һ��Ӧ���Ƶ�Ca��OH��2 | |
| D�� | 25��ʱ��pH=3��HCl��Һ��pH=11��NaOH��Һ�У�H2O�ĵ���̶���ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڢܢ� | B�� | �٢ڢ� | C�� | �٢ڢܢ� | D�� | ȫ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com