
£Ø14·Ö£©ŃĒĀČĖįÄĘ£ØNaClO2£©ŹĒŅ»ÖÖÖŲŅŖµÄŗ¬ĀČĻū¶¾¼Į£¬Ö÷ŅŖÓĆÓŚĖ®µÄĻū¶¾ŅŌ¼°É°ĢĒ”¢ÓĶÖ¬µÄĘÆ°×Óėɱ¾ś”£ŅŌĻĀŹĒ¹żŃõ»ÆĒā·ØÉś²śŃĒĀČĖįÄĘµÄ¹¤ŅÕĮ÷³ĢĶ¼£ŗ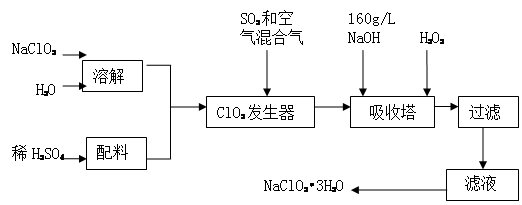
ŅŃÖŖ£ŗ¢ŁNaClO2µÄČܽā¶ČĖęĪĀ¶ČÉżø߶ųŌö“ó£¬ŹŹµ±Ģõ¼žĻĀæɽį¾§Īö³öNaClO2?3H2O”£
¢Ś“æClO2Ņ×·Ö½ā±¬ÕØ£¬Ņ»°ćÓĆĻ”ÓŠĘųĢå»ņæÕĘųĻ”ŹĶµ½10£„ŅŌĻĀ°²Č«”£
¢Ū160 g/L NaOHČÜŅŗŹĒÖø160 gNaOH¹ĢĢåČÜÓŚĖ®ĖłµĆČÜŅŗµÄĢå»żĪŖ1L”£
£Ø1£© 160 g/L NaOHČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ”””””” ”””””£
£Ø2£©·¢ÉśĘ÷ÖŠ¹ÄČėæÕĘųµÄ×÷ÓĆæÉÄÜŹĒ””””””£ØŃ”ĢīŠņŗÅ£©”£
a£®½«SO2Ńõ»Æ³ÉSO3£¬ŌöĒæĖįŠŌ£»
b£®Ļ”ŹĶClO2ŅŌ·ĄÖ¹±¬ÕØ£»
c£®½«NaClO3Ńõ»Æ³ÉClO2
£Ø3£©ĪüŹÕĖžÄŚµÄ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ”””””””””””””””””””””””””””””””””””””£
ĪüŹÕĖžµÄĪĀ¶Č²»Äܳ¬¹ż20”ę£¬ĘäÄæµÄŹĒ”””” ”£
£Ø4£©ŌŚ¼īŠŌČÜŅŗÖŠNaClO2±Č½ĻĪČ¶Ø£¬ĖłŅŌĪüŹÕĖžÖŠÓ¦Ī¬³ÖNaOHÉŌ¹żĮ棬Ŋ¶ĻNaOHŹĒ·ń¹żĮæµÄ¼ņµ„ŹµŃé·½·ØŹĒ ”£
£Ø5£©ĪüŹÕĖžÖŠĪŖ·ĄÖ¹NaClO2±»»¹Ō³ÉNaCl£¬ĖłÓĆ»¹Ō¼ĮµÄ»¹ŌŠŌÓ¦ŹŹÖŠ”£³żH2O2
Ķā£¬»¹æÉŅŌŃ”ŌńµÄ»¹Ō¼ĮŹĒ”” ””””£ØŃ”ĢīŠņŗÅ£©”£
a£®Na2O2 b£®Na2S c£®FeCl2
£Ø6£© “ÓĀĖŅŗÖŠµĆµ½NaClO2?3H2O“Ö¾§ĢåµÄŹµŃé²Ł×÷ŅĄ“ĪŹĒ””””””””””£ØŃ”ĢīŠņŗÅ£©”£
a£®ÕōĮó b£®Õō·¢ c£®×ĘÉÕ d£®¹żĀĖ e£®ĄäČ“½į¾§
ŅŖµĆµ½øü“æµÄNaClO2?3H2O¾§Ģå±ŲŠė½ųŠŠµÄ²Ł×÷ŹĒ”””””””””” ””””£ØĢī²Ł×÷Ćū³Ę£©
£Ø1£© 4mol/L£Ø2·Ö£© £Ø2£© b£Ø2·Ö£©
£Ø3£©2NaOH+2ClO2+H2O2 £½2 NaClO2+2H2O+O2£Ø2·Ö£©£»·ĄÖ¹H2O2·Ö½ā£Ø2·Ö£©
£Ø4£©Į¬Šų²ā¶ØĪüŹÕĖžÄŚČÜŅŗµÄpH£Ø2·Ö£©
£Ø5£© a £Ø1·Ö£© £Ø6£© b”¢e”¢d£ØČ«¶Ō2·Ö£¬ÓŠ“ķ²»µĆ·Ö£©£¬ÖŲ½į¾§£Ø1·Ö£©
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©ÅضČg/L±ķŹ¾1LČÜŅŗÖŠĖłŗ¬ČÜÖŹÖŹĮæµÄ¶ąÉŁ£®160g/LNaOHČÜŅŗ±ķŹ¾1LĒāŃõ»ÆÄĘČÜŅŗŗ¬ÓŠ160gNaOH£®ĮīČÜŅŗĢå»żĪŖ1L£¬Ōņ160gNaOHµÄĪļÖŹµÄĮæĪŖ160g”Ā40g/mo=4mol£®ĖłŅŌøĆČÜŅŗĒāŃõ»ÆÄʵÄĪļÖŹµÄĮæÅضČc£ØNaOH£©=4mol”Ā1L==4mol?L?1”£
£Ø2£©ÓÉŠÅĻ¢¢ŚæÉÖŖ£¬“æClO2Ņ×·Ö½ā±¬ÕØ£¬Ņ»°ćÓĆĻ”ÓŠĘųĢå»ņæÕĘųĻ”ŹĶµ½10%ŅŌĻĀ°²Č«£®·¢ÉśĘ÷ÖŠ¹ÄČėæÕĘųµÄ×÷ÓĆÓ¦ŹĒĻ”ŹĶClO2ŅŌ·ĄÖ¹±¬ÕØ£¬¹ŹŃ”£ŗb”£
£Ø3£©øł¾ŻĮ÷³ĢŠÅĻ¢æÉÖŖ£¬ĪüŹÕĖžÄŚÉś³ÉNaClO2£¬ĖłŅŌŅ»¶ØÓŠClO2”śNaClO2£¬»ÆŗĻ¼Ū½µµĶ£¬±»»¹Ō£»ŌņH2O2±Ų¶Ø±»Ńõ»Æ£¬ÓŠŃõĘų²śÉś£¬·“Ó¦·½³ĢŹ½ĪŖ2NaOH+2ClO2+H2O2=2 NaClO2+2H2O+O2£»H2O2²»ĪČ¶Ø£¬ĪĀ¶Č¹żøߣ¬H2O2ČŻŅ×·Ö½ā£¬ĪüŹÕĖžµÄĪĀ¶Č²»Äܳ¬¹ż20”ę£¬ĘäÄæµÄŹĒ·ĄÖ¹H2O2·Ö½ā”£
£Ø4£©NaOH¹żĮæŌņČÜŅŗ³Ź¼īŠŌ£¬µ«æ¼ĀĒµ½øĆČÜŅŗÓŠĒæŃõ»ÆŠŌ£¬Ń”ŌńÖøŹ¾¼Į»ņpHŹŌÖ½¼ģŃé»įÓöµ½Ńõ»ÆĶŹÉ«ĪŹĢā£¬¹ŹÉś²śÖŠÖ÷ŅŖŹĒÓĆpH¼ĘĮ¬Šų²ā¶ØČÜŅŗpH”£
£Ø5£©»¹ŌŠŌŅŖŹŹÖŠ£®»¹ŌŠŌĢ«Ē棬»į½«ClO2»¹ŌĪŖøüµĶ¼ŪĢ¬²śĪļ£¬Ó°ĻģNaClO2Éś²ś£»·½±ćŗóŠų·ÖĄėĢį“棬¼ÓČėŹŌ¼Į²»ÄÜøÉČÅŗóŠųÉś²ś£®Na2O2ČÜÓŚĖ®Ļąµ±ÓŚH2O2£®Na2S”¢FeCl2»¹ŌŠŌ½ĻĒæ£¬Éś³ÉĪļÓėNaClO2·ÖĄė±Č½ĻĄ§ÄŃ£¬¹ŹŃ”£ŗa”£
£Ø6£©“ÓČÜŅŗÖŠµĆµ½ŗ¬½į¾§Ė®µÄ¾§Ģ壬ֻÄܲÉČ”Õō·¢”¢ÅØĖõ”¢ĄäČ“½į¾§·½·Ø£¬Ķعż¹żĀĖµĆµ½“Ö¾§Ģ壮ĖłŅŌ²Ł×÷Ė³ŠņĪŖbed£»µĆµ½µÄ“Ö¾§Ģå¾¹żÖŲ½į¾§æɵƵ½“æ¶Čøüøߵľ§Ģ唣
æ¼µć£ŗ±¾Ģāæ¼²é»ÆѧĮ÷³ĢµÄ·ÖĪö”¢ĪļÖŹµÄĮæÅØ¶Č”¢»ł±¾²Ł×÷”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĮņĖįŹĒÓĆĶ¾¹ć·ŗµÄ»Æ¹¤ŌĮĻ£¬æÉ×÷ĶŃĖ®¼Į”¢ĪüĖ®¼Į”¢Ńõ»Æ¾£ŗĶ“߻ƼĮµČ”£
¹¤ŅµÖĘĮņĖįĶµÄ·½·Øŗܶą”£
£Ø1£©·½·ØŅ»”¢ÓĆÅØĮņĖįŗĶĶÖĘČ”ĮņĖįĶ”£øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ_____________________,“Ė·ØµÄ×ī“óȱµćŹĒ____________________________”£
£Ø2£©·½·Ø¶ž”¢ÓĆĻ”ĮņĖį”¢ĶŗĶŃõ»ÆĢśÖĘČ”ĮņĖįĶ£¬Éś²śµÄÖ÷ŅŖ¹ż³ĢČēĻĀĶ¼ĖłŹ¾£ŗ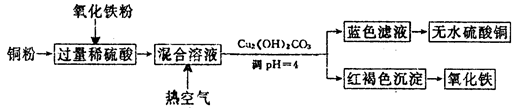
¢ŁĻ”ĮņĖį”¢ĶŗĶŃõ»ÆĢś·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ__________________”¢________________£»
Ļņ»ģŗĻČÜŅŗÖŠĶØČėČČæÕĘųµÄ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ_______________________________”£
¢ŚĒėĖµ³öµ÷ÕūPHĪŖ4µÄÄæµÄŹĒ_______________________£»ÓÉĀĖŅŗµĆµ½ĪŽĖ®ĮņĖįĶµÄŹµŃé²Ł×÷ŹĒ______________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĪŹ“šĢā
£Ø15·Ö£©
Ä³Ń§Ļ°Š”×éŅĄ¾ŻSO2¾ßÓŠ»¹ŌŠŌ£¬ĶĘ²āSO2Äܱ»Cl2Ńõ»ÆÉś³ÉSO2Cl2”£
²éŌÄ׏ĮĻ£ŗSO2Cl2³£ĪĀĻĀĪŖĪŽÉ«ŅŗĢ壬¼«Ņ×Ė®½ā£¬Óö³±ŹŖæÕĘų»į²śÉś°×Īķ”£
¢ń£®»ÆŗĻĪļSO2Cl2ÖŠSŌŖĖŲµÄ»ÆŗĻ¼ŪŹĒ ”£
¢ņ£®ÓƶžŃõ»ÆĆĢŗĶÅØŃĪĖįÖĘĀČĘųµÄ»Æѧ·½³ĢŹ½ŹĒ ”£
¢ó£®ŌŚŹÕ¼ÆĀČĘųĒ°£¬Ó¦ŅĄ“ĪĶعżŹ¢ÓŠ±„ŗĶŹ³ŃĪĖ®ŗĶ µÄĻ“ĘųĘ攣
¢ō£®ÓĆČēĶ¼ĖłŹ¾×°ÖĆŹÕ¼ÆĀśCl2£¬ŌŁĶØČėSO2£¬¼ÆĘųĘæÖŠĮ¢¼“²śÉśĪŽÉ«ŅŗĢ壬
³ä·Ö·“Ó¦ŗ󣬽«ŅŗĢåŗĶŹ£ÓąĘųĢå·ÖĄė£¬½ųŠŠČēĻĀŃŠ¾æ”£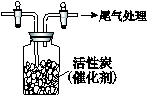
£Ø1£©ŃŠ¾æ·“Ó¦µÄ²śĪļ”£ĻņĖłµĆŅŗĢåÖŠ¼ÓĖ®£¬³öĻÖ°×Īķ£¬Õńµ“”¢¾²ÖƵƵ½ĪŽÉ«ČÜŅŗ”£¾¼ģŃéøĆČÜŅŗÖŠµÄŅõĄė×ӣسżOH£Ķā£©Ö»ÓŠSO42£”¢Cl£ £¬Ö¤Ć÷ĪŽÉ«ŅŗĢåŹĒSO2Cl2”£
¢Ł Š“³öSO2Cl2ÓėH2O·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
¢Ś ¼ģŃéøĆČÜŅŗÖŠCl£µÄ·½·ØŹĒ ”£
£Ø2£©¼ĢŠųŃŠ¾æ·“Ó¦½ųŠŠµÄ³Ģ¶Č”£ÓĆNaOHČÜŅŗĪüŹÕ·ÖĄė³öµÄĘųĢ壬ÓĆĻ”ŃĪĖįĖį»Æŗó£¬ŌŁµĪ¼ÓBaCl2ČÜŅŗ£¬²śÉś°×É«³Įµķ”£
¢Ł øĆ°×É«³ĮµķµÄ³É·ÖŹĒ ”£
¢Ś Š“³öSO2ÓėCl2·“Ó¦µÄ»Æѧ·½³ĢŹ½£¬²¢²ūŹöĄķÓÉ______”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
ij½šŹōMµÄĒāŃõ»ÆĪļµÄĖ®ŗĻ¾§Ģå[M(OH)2?xH2O]ÓėNa2CO3»ģŗĻĪļ¹²36.800g£¬¼ÓČė×ćĮæµÄĖ®ŗó£¬Éś³ÉMCO3µÄ°×É«³Įµķ£¬½«³ĮµķĀĖ³ö£¬Ļ“¾»ŗęøÉ£¬ĘäÖŹĮæĪŖ9.850g”£
47. ½« 9.850g MCO3øßĪĀ×ĘČČÖĮŗćÖŲ£¬µĆµ½7.650g MO¹ĢĢ壬Ōņ²śÉśCO2ĘųĢå_______mol”£
48£®ĀĖŅŗÓėĖį×÷ÓĆ²»²śÉśĘųĢ壻ČōÓĆ×ćĮæµÄļ§ŃĪÓėĀĖŅŗ¹²ČČ£¬Ōņ²śÉś4.48LĘųĢå(±ź×¼×“æö)£¬ĀĖŅŗÖŠOH?µÄĪļÖŹµÄĮæĪŖ_______mol”£
49.MµÄĻą¶ŌŌ×ÓÖŹĮæĪŖ_________£»ŹŌĶعż¼ĘĖćČ·¶ØM(OH)2?xH2OÖŠxµÄÖµ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ijŠ£»ÆѧŠ”×éĪŖĢ½¾æĢśÓėÅØĮņĖį·“Ó¦ŹĒ·ńÉś³ÉSO2£¬Éč¼ĘĮĖŅŌĻĀ×°ÖĆ½ųŠŠŹµŃ锣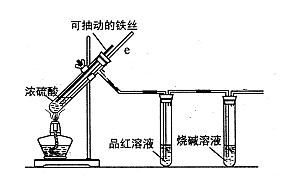
£Ø1£©Š“³öĢśÓėÅØĮņĖį·“Ó¦µÄ»Æѧ·½³ĢŹ½ £»
ČōŌŚČ”ÓĆÅØĮņĖįµÄ¹ż³ĢÖŠ£¬²»É÷ŌŚĘ¤·ōÉĻÕ“ÉŁĮæÅØĮņĖį£¬“¦ĄķµÄ·½·ØŹĒ ”£
£Ø2£©µ¼Ęų¹ÜeÓŠĮ½øöÖ÷ŅŖ×÷ÓĆ£ŗŅ»ŹĒŌŚ·“Ó¦¹ż³ĢÖŠ£¬Ņņµ¼¹Ü²åČėŅŗĆęĻĀ£¬æÉĘšµ½”°Ņŗ·ā”±×÷ÓĆ×čÖ¹SO2ĘųĢåŅŻ³ö¶ų·ĄÖ¹ĪŪČ¾»·¾³£»¶žŹĒ ”£
£Ø3£©Ę·ŗģČÜŅŗµÄ×÷ÓĆŹĒ ”£
£Ø4£©Š”×éĢÖĀŪŗóČĻĪŖ£ŗÓÉÓŚ³£ÓƵÄĢśĖæŹĒĢśĢ¼ŗĻ½š£¬Éś³ÉµÄĘųĢåÖŠ»¹æÉÄÜŗ¬ÓŠCO2”£Š“³öÉś³ÉCO2µÄ»Æѧ·½³ĢŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
£Ø9·Ö£©Ņ»¶ØĮæµÄÅØĮņĖįÓė×ćĮæZn³ä·Ö·“Ó¦Ź±ÓŠSO2ŗĶH2Éś³É”£Ä³Š£»Æѧъ¾æŠŌѧĻ°Š”×é“Ӷ؊Ō·½Ćę¶Ō“Ė×÷ĮĖŃŠ¾æ”£
°“Ķ¼×é×°ŗĆŹµŃé×°ÖĆ£¬Ī¢ČČŹŌ¹ÜA£¬¹Ū²ģµ½C”¢D”¢EÖŠ¾łÓŠĘųÅŻ²śÉś£»ĖęŗóĘųÅŻĮæ¼õÉŁ£¬Ę·ŗģČÜŅŗĶŹÉ«£¬DÖŠĻČ³öĻÖ»ė×Ē£¬ŗó»ė×ĒĻūŹ§£»·“Ó¦½Ļ³¤Ź±¼äŗó£¬C”¢D”¢EÖŠµÄĘųÅŻĮæÓÖ»įĆ÷ĻŌŌö¼Ó”£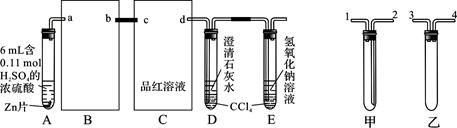
ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©“Ó¼×”¢ŅŅÖŠŃ”ŌńŗĻŹŹµÄ×°ÖĆĢīČėB”¢CÖŠ£¬²¢½ųŠŠÕżČ·Į¬½Ó£¬a½Ó______”¢______½Ób£¬c½Ó______”¢______½Ód£»D”¢EĮ½Ö§ŹŌ¹ÜÖŠCCl4µÄ×÷ÓĆŹĒ________________________”£
£Ø2£©ÄÜÖ¤Ć÷ÅØĮņĖį¾ßÓŠĒæŃõ»ÆŠŌµÄŹµŃéĻÖĻóĪŖ_______________________________£»ŹµŃé¹ż³ĢÖŠ£¬ÅØĮņĖį±ķĻÖĒæŃõ»ÆŠŌµÄ·“Ó¦·½³ĢŹ½ŹĒ£ŗ
____________________________________________________________________
£Ø3£©·“Ó¦½Ļ³¤Ź±¼äŗóĘųÅŻĮæÓÖ»įĆ÷ĻŌŌö¼ÓµÄŌŅņŹĒ________________________________
____________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
£Ø16·Ö£©»šĮ¦·¢µēŌŚĪŅ¹śµÄÄÜŌ“ĄūÓĆÖŠÕ¼½Ļ“ó±ČÖŲ£¬µ«ŹĒÅŷųöµÄSO2»įŌģ³ÉŅ»ĻµĮŠ»·¾³ŗĶÉśĢ¬ĪŹĢā£¬Ö±½ÓÅÅ·Åŗ¬SO2µÄŃĢĘų»įŠĪ³ÉĖįÓź£¬Ī£ŗ¦»·¾³”£
£Ø1£©ÓĆ»Æѧ·½³ĢŹ½±ķŹ¾SO2ŠĪ³ÉĮņĖįŠĶĖįÓźµÄ·“Ó¦£ŗ ”£2·Ö
£Ø2£©¹¤ŅµÉĻÓĆNa2SO3ČÜŅŗĪüŹÕŃĢĘųÖŠµÄSO2”£½«ŃĢĘųĶØČė1.0 mol”¤L-1µÄNa2SO3ČÜŅŗ£¬ČÜŅŗpH²»¶Ļ¼õŠ””£µ±ČÜŅŗpHŌ¼ĪŖ6Ź±£¬ĪüŹÕSO2µÄÄÜĮ¦ĻŌÖųĻĀ½µ£¬Ó¦øü»»ĪüŹÕ¼Į”£
¢Ł “ĖŹ±ČÜŅŗÖŠc(SO32ØC)µÄÅØ¶ČŹĒ0.2 mol”¤L-1£¬ŌņČÜŅŗÖŠc(HSO3ØC)ŹĒ_______mol?L-1”£
¢Ś ĻņpHŌ¼ĪŖ6µÄĪüŹÕ¼ĮÖŠĶØČė×ćĮæµÄO2£¬æɽ«ĘäÖŠµÄNaHSO3×Ŗ»ÆĪŖĮ½ÖÖĪļÖŹ£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ ”£2·Ö
¢Ū Ä³ŃŠ¾æŠ”×éĪŖĢ½¾æĢįøßŗ¬ĮņŃĢĘųÖŠSO2µÄĪüŹÕŠ§ĀŹµÄ“ėŹ©£¬Ä£ÄāŹµŃéĪüŹÕŗ¬ĮņŃĢĘų£¬ŹµŃé½į¹ūČēĶ¼ĖłŹ¾”£Ōņ£ŗ £¬ÓŠĄūÓŚĢįøßSO2µÄĪüŹÕŠ§ĀŹ”£2·Ö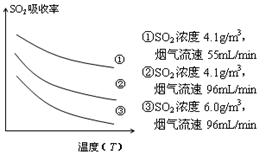
£Ø3£©¹¤³§²Öæā“ę·ÅµÄNa2SO3Ņ©Ę·ŅŃ²æ·Ö±»æÕĘųŃõ»Æ£¬øĆ»ÆѧŠ”×éĻėÓĆŅŃÖŖÅØ¶ČµÄĖįŠŌKMnO4ČÜŅŗĄ“Č·¶ØĘäŗ¬Į棬¾ßĢå²½ÖčČēĻĀ£ŗ
²½Öči””³ĘȔѳʷ1.000 g”£
²½Öčii””½«ŃłĘ·Čܽāŗó£¬ĶźČ«×ŖŅʵ½250 mLČŻĮæĘæÖŠ£¬¶ØČŻ£¬³ä·ÖŅ”ŌČ”£
²½Öčiii””ŅĘČ”25.00 mLѳʷČÜŅŗÓŚ250 mL׶ŠĪĘæÖŠ£¬ÓĆ0.01000 mol”¤L£1 KMnO4±ź×¼ČÜŅŗµĪ¶ØÖĮÖÕµć”£
°“ÉĻŹö²Ł×÷·½·ØŌŁÖŲø“2“Ī”£
¢Ł Š“³ö²½ÖčiiiĖł·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½_________________________________£»
¢Ś ŌŚÅäÖĘ0.01000 mol”¤L£1 KMnO4ČÜŅŗŹ±ČōŃöŹÓ¶ØČŻ£¬Ōņ×īÖÕ²āµĆŅ©Ę·ÖŠNa2SO3µÄŗ¬Įæ________(Ģī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°ĪŽÓ°Ļģ”±)”£
¢Ū ijĶ¬Ń§Éč¼ĘÓĆĻĀĮŠŅĒĘ÷½ųŠŠµĪ¶ØŹµŃé(¼Š³Ö²æ·ÖĀŌČ„)£¬×īŗĻĄķµÄ×éŗĻŹĒ (Ģī×ÖÄø)”£

A B C D E
¢Ü µĪ¶Ø½į¹ūČēĻĀ±ķĖłŹ¾£ŗ
| µĪ¶Ø“ĪŹż | “ż²āČÜŅŗ µÄĢå»ż/mL | ±ź×¼ČÜŅŗµÄĢå»ż | |
| µĪ¶ØĒ°æĢ¶Č/mL | µĪ¶ØŗóæĢ¶Č/mL | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 2.20 | 20.20 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
(12·Ö)”¾»Æѧ”Ŗ”Ŗ»ÆѧÓė¼¼Źõ”æ
¹¤ŅµÉĻŅŌ»ĘĢśæóĪŖŌĮĻ£¬²ÉÓĆ½Ó“„·ØÉś²śĮņĖį”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ģŃÉÕ»ĘĢśæóµÄÉč±øĆū³ĘŹĒ__________£¬½ųĮĻĒ°±ŲŠė½«»ĘĢśæó·ŪĖ飬ÄæµÄŹĒ________”£ĖłµĆĀÆŌüµÄÓĆĶ¾ÓŠ__________________________(ĢīŅ»ÖÖÓĆĶ¾)”£
£Ø2£©½ųČė½Ó“„ŹŅĒ°µÄĘųĢå±ŲŠė¾¹ż¾»»Æ“¦Ąķ£¬ÄæµÄŹĒ·ĄÖ¹________________________________”£
£Ø3£©Éś²ś¹ż³ĢÖŠ£¬ĪüŹÕČżŃõ»ÆĮņ³£ÓƵÄĪļÖŹŹĒ_____________________________”£
£Ø4£©ĪĀ¶Č±ä»ÆÓė¶žŃõ»ÆĮņ×Ŗ»ÆĀŹµÄ±ä»Æ¹ŲĻµæÉÓĆĻĀĶ¼ÖŠµÄĒśĻß______(Ģī”°a”±»ņ”°b”±)±ķŹ¾”£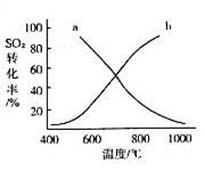
£Ø5£©¹¤ŅµÉĻ³£ÓĆŹģŹÆ»ŅŗĶĮņĖį“¦Ąķ”¢»ŲŹÕĪ²ĘųÖŠµÄÓŠŗ¦ĘųĢ唣·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ
________________________________Ӣ_________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
Ä³Ń§ÉśĄūÓĆŅŌĻĀ×°ÖĆĢ½¾æĀČĘųÓė°±ĘųÖ®¼äµÄ·“Ó¦”£ĘäÖŠA”¢F·Ö±šĪŖ°±ĘųŗĶĀČĘųµÄ·¢Éś×°ÖĆ£¬CĪŖ“æ¾»øÉŌļµÄĀČĘųÓė°±Ęų·“Ó¦µÄ×°ÖĆ”£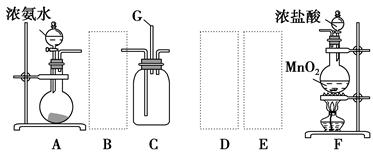
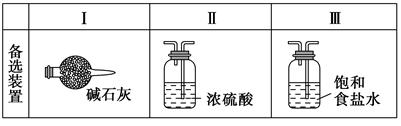
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)×°ÖĆFÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ ”£
(2)×°ÖĆAÖŠµÄÉÕĘæÄŚ¹ĢĢåæÉŃ”ÓĆ (Ń”ĢīŅŌĻĀŃ”ĻīµÄ“śŗÅ)”£
A£®¼īŹÆ»Ņ
B£®ÉśŹÆ»Ņ
C£®¶žŃõ»Æ¹č
D£®ĪåŃõ»Æ¶žĮ×
E£®ÉÕ¼ī
(3)ŠéĻßæņÄŚÓ¦Ģķ¼Ó±ŲŅŖµÄ³żŌÓ×°ÖĆ£¬Ēė“ÓÉĻĶ¼µÄ±øŃ”×°ÖĆÖŠŃ”Ōń£¬²¢½«±ąŗÅĢīČėĻĀĮŠæÕøń£ŗB £¬D £¬E ”£(¾łĢī±ąŗÅ)
(4)ĀČĘųŗĶ°±ĘųŌŚ³£ĪĀĻĀĻą»ģ¾Ķ»į·“Ӧɜ³ÉĀČ»Æļ§ŗĶµŖĘų£¬×°ÖĆCÄŚ³öĻÖÅØŗńµÄ°×ŃĢ²¢ŌŚČŻĘ÷ÄŚ±ŚÄż½į£¬ĒėÉč¼ĘŹµŃé·½°ø¼ų¶ØøĆ¹ĢĢå¾ĶŹĒĀČ»Æļ§£ŗ ”£
(5)“Ó×°ÖĆCµÄ³öĘų¹ÜæŚ“¦ŅŻ³öµÄĪ²ĘųæÉÄÜŗ¬ÓŠĪŪČ¾»·¾³µÄĘųĢ壬ČēŗĪ“¦Ąķ£æ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com