
ЁОЬтФПЁПAЁЂBЁЂCЁЂD ОљЮЊЖЬжмЦкдЊЫизщГЩЕФЮяжЪЃЌЫќУЧжЎМфЗћКЯШчЯТзЊЛЏЙиЯЕЃК
![]()
ЃЈ1ЃЉШєA ЮЊПЩЪЙЪЊШѓЕФКьЩЋЪЏШяЪджНБфРЖЕФЦјЬЌЧтЛЏЮяЃЌX ЮЊЫЎЃЌD ЮЊживЊЕФЛЏЙЄдСЯЁЃ
Ђй A ЕФЕчзгЪНЮЊ_________________ЃЌAЁњB ЕФЛЏбЇЗНГЬЪНЮЊ______________________ЁЃ
ЂкA КЭ D ЗЂЩњЛЏКЯЗДгІЕУЕНЕФЛЏКЯЮя E ЕФЛЏбЇЪНЪЧ___________ЃЌгУРызгЗНГЬЪНБэЪОМьбщEжабєРызгЕФЗНЗЈ________________ЁЃ
ЂлаДГіDЕФЯЁШмвКгыCuЗДгІЕФРызгЗНГЬЪН____________________________ЁЃ
ЃЈ2ЃЉШєAЮЊЗЧН№ЪєЕЅжЪЃЌXЮЊЫЎЃЌИУзЊЛЏЙиЯЕЮЊЙЄвЕЩњВњDЕФвЛЯЕСаЗДгІЁЃ
ЂйНЋBЭЈШыфхЫЎжабеЩЋЭЪШЅЃЌЬхЯжСЫBЕФ___________________адЃЌаДГіИУБфЛЏЕФЛЏбЇЗНГЬЪН________________________ЁЃ
ЂкAКЭH2ЗЂЩњЛЏКЯЗДгІЕУЕНЕФEЃЌдкBгыEЕФЗДгІжаЃЌбѕЛЏВњЮяКЭЛЙдВњЮяЕФжЪСПБШЮЊ_______________________________ЁЃ
ЂлаДГіDЕФХЈШмвКгыCuЗДгІЕФЛЏбЇЗНГЬЪН_________________________________________________________ЁЃ
ЃЈ3ЃЉШєAЮЊЬўЕФКЌбѕбмЩњЮяЃЌвНСЦЩЯГЃгУ75%ЃЈЬхЛ§ЗжЪ§ЃЉAЕФЫЎШмвКзїЯћЖОМСЃЌXЮЊМзДМЃЈCH3OHЃЉЁЃ
ЂйAЁњBЕФЛЏбЇЗНГЬЪНЮЊ____________________________ЃЛCЁњDЕФЛЏбЇЗНГЬЪНЮЊ_____________________________________ЁЃ
ЂкЯТСаЫЕЗЈе§ШЗЕФЪЧ___________________________________ЃЈбЁЬюађКХзжФИЃЉ
a. A ПЩЭЈЙ§ЦЯЬбЬЧЗжНтЗДгІЕУЕН b. B ЕФЙйФмЭХЕФНсЙЙМђЪНЮЊ-COH
c. C ПЩгы NaHCO3 ЗДгІВњЩњ CO2 d. A гыX ЛЅЮЊЭЌЯЕЮя
ЂлвбжЊЃКCHЁдCH дквЛЖЈЬѕМўЯТПЩгыCЗДгІЕУЕНEЃЈНсЙЙМђЪНЮЊ CH2=CHOOCCH3ЃЉЃЌдђЩЯЪіЗДгІЕФЗДгІРраЭЮЊ_______________________ЃЌEжаЕФЙйФмЭХУћГЦЮЊ_____________ЃЌEЗЂЩњМгОлЗДгІЕФЛЏбЇЗНГЬЪНЮЊ_____________________________ЁЃ
ЁОД№АИЁП![]() 4NH3+5O2
4NH3+5O2![]() 4NO+6H2O NH3+HNO3=NH4NO3 NH4++OH-
4NO+6H2O NH3+HNO3=NH4NO3 NH4++OH-![]() NH3Ёќ+H2O 3Cu+8H++2NO3-=3Cu2+ +2NOЁќ+4H2O ЛЙд SO2+Br2+2H2O=2HBr+H2SO4 2:1 Cu+2H2SO4(ХЈ)
NH3Ёќ+H2O 3Cu+8H++2NO3-=3Cu2+ +2NOЁќ+4H2O ЛЙд SO2+Br2+2H2O=2HBr+H2SO4 2:1 Cu+2H2SO4(ХЈ)![]() CuSO4+SO2Ёќ+2H2O 2CH3CH2OH+O2
CuSO4+SO2Ёќ+2H2O 2CH3CH2OH+O2![]() 2CH3CHO+2H2O CH3OH+CH3COOH
2CH3CHO+2H2O CH3OH+CH3COOH![]() CH3COOCH3+H2O acd МгГЩЗДгІ ѕЅЛљКЭЬМЬМЫЋМќ nCH2=CHOOCCH3
CH3COOCH3+H2O acd МгГЩЗДгІ ѕЅЛљКЭЬМЬМЫЋМќ nCH2=CHOOCCH3![]()
![]()
ЁОНтЮіЁП
(1)ШєA ЮЊПЩЪЙЪЊШѓЕФКьЩЋЪЏШяЪджНБфРЖЕФЦјЬЌЧтЛЏЮяЃЌдђAЮЊNH3ЃЌX ЮЊЫЎЃЌBЮЊNOЃЌCЮЊNO2ЃЌDЮЊHNO3ЃЌОнДЫЗжЮіНтД№ЃЛ
(2)ШєAЮЊЗЧН№ЪєЕЅжЪЃЌXЮЊЫЎЃЌНЋBЭЈШыфхЫЎжабеЩЋЭЪШЅЃЌдђAЮЊSЃЌBЮЊSO2ЃЌCЮЊSO3ЃЌDЮЊH2SO4ЃЌОнДЫЗжЮіНтД№ЃЛ
(3)ШєAЮЊЬўЕФКЌбѕбмЩњЮяЃЌвНСЦЩЯГЃгУ75%(ЬхЛ§ЗжЪ§)AЕФЫЎШмвКзїЯћЖОМСЃЌдђAЮЊввДМ(CH3CH2OH)ЃЌXЮЊМзДМ(CH3OH)ЃЌдђBЮЊввШЉЃЌCЮЊввЫсЃЌDЮЊввЫсМзѕЅЃЌОнДЫЗжЮіНтД№ЁЃ
(1)ШєA ЮЊПЩЪЙЪЊШѓЕФКьЩЋЪЏШяЪджНБфРЖЕФЦјЬЌЧтЛЏЮяЃЌдђAЮЊNH3ЃЌX ЮЊЫЎЃЌBЮЊNOЃЌCЮЊNO2ЃЌDЮЊHNO3ЃЌ
ЂйA ЕФЕчзгЪНЮЊ![]() ЃЌAЁњB АБЦјЕФДпЛЏбѕЛЏЃЌЛЏбЇЗНГЬЪНЮЊ4NH3+5O2
ЃЌAЁњB АБЦјЕФДпЛЏбѕЛЏЃЌЛЏбЇЗНГЬЪНЮЊ4NH3+5O2![]() 4NO+6H2OЃЛ
4NO+6H2OЃЛ
ЂкNH3КЭHNO3ЗЂЩњЛЏКЯЗДгІЕУЕНЕФЛЏКЯЮяЯѕЫсяЇЃЌЛЏбЇЪНЪЧNH3+HNO3=NH4NO3ЃЌМьбщяЇИљРызгЕФЗНЗЈШЁЩйСПД§ВтвКЬхгкЪдЙмжаЃЌЕЮМгЧтбѕЛЏФЦШмвКВЂМгШШЃЌгУЪЊШѓЕФКьЩЋЪЏШяЪджНгкЪдЙмПкЃЌЪджНБфРЖЃЌжЄУїгаАБЦјЩњГЩЃЌНјЖјжЄУїгаяЇИљРызгДцдкЃЌЗЂЩњЕФРызгЗДгІЮЊЃКNH4++OH-![]() NH3Ёќ+H2OЃЛ
NH3Ёќ+H2OЃЛ
ЂлЯЁЯѕЫсгыCuЗДгІЩњГЩЯѕЫсЭЁЂвЛбѕЛЏЕЊКЭЫЎЃЌРызгЗНГЬЮЊ3Cu+8H++2NO3-=3Cu2+ +2NOЁќ+4H2OЃЛ
(2) ШєAЮЊЗЧН№ЪєЕЅжЪЃЌXЮЊЫЎЃЌНЋBЭЈШыфхЫЎжабеЩЋЭЪШЅЃЌдђAЮЊSЃЌBЮЊSO2ЃЌCЮЊSO3ЃЌDЮЊH2SO4ЃЛ
ЂйНЋSO2ЭЈШыфхЫЎжаЗЂЩњбѕЛЏЛЙдЗДгІЩњГЩСђЫсКЭфхЛЏЧтЃЌфхЫЎбеЩЋЭЪШЅЃЌЬхЯжСЫSO2ЕФЛЙдадЃЌИУБфЛЏЕФЛЏбЇЗНГЬЪНSO2+Br2
ЂкSКЭH2ЗЂЩњЛЏКЯЗДгІЕУЕНЕФH2SЃЌдкH2SгыSO2ЕФЗДгІжаЩњГЩSКЭЫЎЃЌЗДгІЗНГЬЪНЮЊЃК2H2S+SO2=3S+2H2OЃЌH2SжаЕФSЛЏКЯМлЩ§ИпЃЌБЛбѕЛЏЃЌSO2жаЕФSдЊЫиЛЏКЯМлНЕЕЭЃЌБЛЛЙдЃЌбѕЛЏВњЮяКЭЛЙдВњЮяЕФжЪСПБШЮЊ2:1ЃЛ
ЂлХЈСђЫсгыCuдкМгШШЬѕМўЯТЗДгІЩњГЩСђЫсЭЁЂЖўбѕЛЏСђКЭЫЎЃЌЛЏбЇЗНГЬЪНCu+2H2SO4(ХЈ)![]() CuSO4+SO2Ёќ+2H2OЃЛ
CuSO4+SO2Ёќ+2H2OЃЛ
(3) ШєAЮЊЬўЕФКЌбѕбмЩњЮяЃЌвНСЦЩЯГЃгУ75%(ЬхЛ§ЗжЪ§)AЕФЫЎШмвКзїЯћЖОМСЃЌЃЌдђAЮЊввДМ(CH3CH2OH)ЃЌXЮЊМзДМ(CH3OH)ЃЌдђBЮЊввШЉЃЌCЮЊввЫсЃЌDЮЊввЫсМзѕЅЃЛ
ЂйввДМдкДпЛЏМСМгШШЬѕМўЯТгыбѕЦјЗЂЩњДпЛЏбѕЛЏЗДгІЃЌЛЏбЇЗНГЬЪНЮЊ2CH3CH2OH+O2![]() 2CH3CHO+2H2OЃЛCЮЊввЫсЃЌXЮЊМзДМ(CH3OH)ЃЌввЫсКЭМзДМдкХЈСђЫсМгШШЬѕМўЯТЗЂЩњѕЅЛЏЗДгІЃЌЛЏбЇЗНГЬЪНЮЊCH3OH+CH3COOH
2CH3CHO+2H2OЃЛCЮЊввЫсЃЌXЮЊМзДМ(CH3OH)ЃЌввЫсКЭМзДМдкХЈСђЫсМгШШЬѕМўЯТЗЂЩњѕЅЛЏЗДгІЃЌЛЏбЇЗНГЬЪНЮЊCH3OH+CH3COOH![]() CH3COOCH3+H2OЃЛ
CH3COOCH3+H2OЃЛ
ЂкaЃЎA ЮЊввДМЃЌЮобѕЛђШБбѕЕФЬѕМўЯТЃЌЭЈЙ§УИЕФДпЛЏзїгУЃЌАбЦЯЬбЬЧЕШгаЛњЮяВЛГЙАйЕзЕФбѕЛЏЗжНтГЩОЦОЋЛђШщЫсЕШЃЌЗДгІЮЊC6H12O6![]() 2C2H5OH+ 2CO2ЃЌЙЪaе§ШЗЃЛ
2C2H5OH+ 2CO2ЃЌЙЪaе§ШЗЃЛ
bЃЎBЮЊввШЉЃЌдђBЕФЙйФмЭХЕФНсЙЙМђЪНЮЊ-CHOЃЌЙЪbДэЮѓЃЛ
cЃЎCЮЊввЫсЃЌввЫсЕФЫсадЧПгкЬМЫсЃЌдђCПЩгыNaHCO3ЗДгІВњЩњCO2ЃЌЙЪcе§ШЗЃЛ
dЃЎНсЙЙЯрЫЦЃЌРрБ№ЯрЭЌЃЌЗжзгзщГЩЩЯЯрВювЛИіЛђЖрИі-CH2-ЕФгаЛњЮяЛЅЮЊЭЌЯЕЮяЃЌAЮЊввДМ(CH3CH2OH)ЃЌXЮЊМзДМ(CH3OH)ЃЌЛЅЮЊЭЌЯЕЮяЃЌЙЪdе§ШЗЃЛ
Д№АИбЁacdЃЛ
ЂлвбжЊЃКCHЁдCH дквЛЖЈЬѕМўЯТПЩгыCЗДгІЕУЕНE(НсЙЙМђЪНЮЊCH2=CHOOCCH3)ЃЌШ§МќБфЫЋМќЃЌЗДгІРраЭЮЊМгГЩЗДгІЃЌEжаЕФЙйФмЭХУћГЦЮЊѕЅЛљКЭЬМЬМЫЋМќЃЌEЗЂЩњМгОлЗДгІЕФЛЏбЇЗНГЬЪНЮЊnCH2=CHOOCCH3![]()
![]() ЁЃ
ЁЃ


 бЇСЗПьГЕЕРПкЫуаФЫуЫйЫуЬьЬьСЗЯЕСаД№АИ
бЇСЗПьГЕЕРПкЫуаФЫуЫйЫуЬьЬьСЗЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЖЬжмЦкдЊЫиRЁЂTЁЂQЁЂWдкжмЦкБэжаЕФЯрЖдЮЛжУШчЭМЫљЪОЃЌЦфжаQдзгЕФжЪзгЪ§ЪЧЦфзюЭтВуЕчзгЪ§ЕФШ§БЖЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧ

A. дзгАыОЖЃКRЃОT
B. бѕЛЏЮяЖдгІЫЎЛЏЮяЕФЫсадЃКWЃОQ
C. зюМђЕЅЦјЬЌЧтЛЏЮяЕФШШЮШЖЈадЃКRЃОQ
D. QгыWаЮГЩЕФЛЏКЯЮяжаЃЌИїдзгЕФзюЭтВуЖМТњзу8ЕчзгЮШЖЈНсЙЙ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЮЊЗжЮіФГбЮЕФГЩЗжЃЌ зіСЫШчЯТЪЕбщЃК
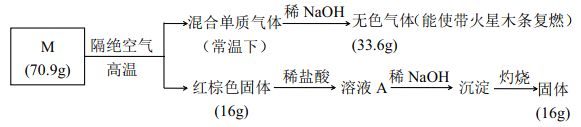
ЧыЛиД№ЃК
ЃЈ1ЃЉбЮ M ЕФЛЏбЇЪНЪЧ_________ЃЛ
ЃЈ2ЃЉБЛ NaOH ЮќЪеЕФЦјЬхЕФЕчзгЪН____________ЃЛ
ЃЈ3ЃЉЯђШмвК A жаЭЈШыH2S ЦјЬхЃЌ гаЕЛЦЩЋГСЕэВњЩњЃЌ аДГіЗДгІЕФРызгЗНГЬЪН________ (ВЛПМТЧПеЦјЕФгАЯь)ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЫЕЗЈВЛе§ШЗЕФЪЧ
A.ХЈСђЫсКЭХЈЯѕЫсЖМОпгаЧПбѕЛЏадЃЌЖМФмАб HCl ЦјЬхбѕЛЏ
B.ЗЧН№ЪєбѕЛЏЮяВЛвЛЖЈЪЧЫсадбѕЛЏЮяЃЌН№ЪєбѕЛЏЮяЖрЪ§ЪЧМюадбѕЛЏЮя
C.ЭЌЮТЭЌбЙЯТЃЌСНЗнЯрЭЌжЪСПЕФаПЗлЃЌЗжБ№гызуСПЕФЯЁСђЫсКЭХЈСђЫсЗДгІЃЌВњЩњЦјЬхЕФЬхЛ§ЯрЭЌ
D.НЋ CO2 ЦјЬхЭЈШыBaCl2 ШмвКжажСБЅКЭЮДМћГСЕэЩњГЩЃЌМЬајЭЈШы NH3 дђгаГСЕэЩњГЩ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТБэжаЪЧAЁЂBЁЂCЁЂDЁЂEЮхжжЖЬжмЦкдЊЫиЕФФГаЉаджЪЃЌЯТСаХаЖЯе§ШЗЕФЪЧ

A. CЁЂDЁЂE ЕФЧтЛЏЮяЕФЮШЖЈадЃКCЃОDЃОE
B. дЊЫи A ЕФдзгзюЭтВуЙьЕРжаЮозда§зДЬЌЯрЭЌЕФЕчзг
C. дЊЫи CЁЂD жЎМфВЛПЩФмаЮГЩЛЏКЯЮя
D. гыдЊЫи B ЭЌжмЦкЧвЕквЛЕчРыФмзюаЁЕФдЊЫиЕФЕЅжЪФмгы H2O ЗЂЩњжУЛЛЗДгІ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвбжЊ298.15KЪБЃЌПЩФцЗДгІ:Pb2+ЃЈaqЃЉ+SnЃЈsЃЉ![]() PbЃЈsЃЉ+Sn2+ЃЈaqЃЉЕФЦНКтГЃЪ§K=2.2ЃЌШєШмвКжаPb2+КЭSn2+ЕФХЈЖШОљЮЊ0.010molЁЄL-1ЃЌдђЗДгІНјааЕФЗНЯђЪЧ
PbЃЈsЃЉ+Sn2+ЃЈaqЃЉЕФЦНКтГЃЪ§K=2.2ЃЌШєШмвКжаPb2+КЭSn2+ЕФХЈЖШОљЮЊ0.010molЁЄL-1ЃЌдђЗДгІНјааЕФЗНЯђЪЧ
A. ЯђгвНјаа B. ЯђзѓНјаа C. ДІгкЦНКтзДЬЌ D. ЮоЗЈХаЖЯ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁП![]() ЪЧвЛжжИпаЇАВШЋЯћЖОМСЃЌГЃЮТЯТ
ЪЧвЛжжИпаЇАВШЋЯћЖОМСЃЌГЃЮТЯТ![]() ЮЊКьЛЦЩЋгаДЬМЄадЦјЮЖЦјЬхЃЌЦфШлЕуЮЊ-59.5ЁцЃЌЗаЕуЮЊ11.0ЁцЃЌФмШмгкЫЎЕЋВЛгыЫЎЗДгІЃЌгіШШЫЎЛКТ§ЫЎНтЁЃФГбаОПадбЇЯАаЁзщгћжЦБИ
ЮЊКьЛЦЩЋгаДЬМЄадЦјЮЖЦјЬхЃЌЦфШлЕуЮЊ-59.5ЁцЃЌЗаЕуЮЊ11.0ЁцЃЌФмШмгкЫЎЕЋВЛгыЫЎЗДгІЃЌгіШШЫЎЛКТ§ЫЎНтЁЃФГбаОПадбЇЯАаЁзщгћжЦБИ![]() ЫЎШмвКВЂМьбщЦфаджЪЁЃ
ЫЎШмвКВЂМьбщЦфаджЪЁЃ
ЂёЃЎЖўбѕЛЏТШЫЎШмвКжЦБИЁЃ

дкдВЕзЩеЦПжаЯШЗХШы![]() ЙЬЬхКЭ
ЙЬЬхКЭ![]() ЃЌШЛКѓдйМгШы5mLЯЁСђЫсЃЌгУДХСІНСАшАєНСАшЃЈШчЭМЃЉЃЌНЋЩеЦПЗХдкШШЫЎдЁжаЃЌБЃГж60Ёц~80ЁцЃЌжСBжаЙуПкЦПФкГЪЩюКьЛЦЩЋЪБЭЃжЙМгШШЁЃЛиД№ЯТСаЮЪЬтЃК
ЃЌШЛКѓдйМгШы5mLЯЁСђЫсЃЌгУДХСІНСАшАєНСАшЃЈШчЭМЃЉЃЌНЋЩеЦПЗХдкШШЫЎдЁжаЃЌБЃГж60Ёц~80ЁцЃЌжСBжаЙуПкЦПФкГЪЩюКьЛЦЩЋЪБЭЃжЙМгШШЁЃЛиД№ЯТСаЮЪЬтЃК
(1)зАжУAгУЫЎдЁМгШШЕФгХЕуЪЧ_________ЃЛзАжУAжаЫЎдЁЮТЖШВЛЕЭгк60ЁцЃЌЦфдвђЪЧ_______________ЁЃ
(2)зАжУAжаЗДгІЩњГЩ![]() МА
МА![]() ЕШВњЮяЕФЛЏбЇЗНГЬЪНЮЊ_____________________ЃЛ
ЕШВњЮяЕФЛЏбЇЗНГЬЪНЮЊ_____________________ЃЛ
(3)зАжУBЕФЫЎжаашЗХШыБљПщЕФФПЕФЪЧ__________________ЃЛвбжЊ![]() ЛКТ§ЫЎНтЩњГЩЕФКЌТШЛЏКЯЮяжЛга
ЛКТ§ЫЎНтЩњГЩЕФКЌТШЛЏКЯЮяжЛга![]() КЭ
КЭ![]() ЃЌЧвЮяжЪЕФСПжЎБШЮЊ2:1ЃЌдђИУЗДгІЕФЛЏбЇЗНГЬЪНЮЊ____________ЃЛзАжУCжаЕФ
ЃЌЧвЮяжЪЕФСПжЎБШЮЊ2:1ЃЌдђИУЗДгІЕФЛЏбЇЗНГЬЪНЮЊ____________ЃЛзАжУCжаЕФ![]() ШмвКЮќЪеЮВЦјжаЕФ
ШмвКЮќЪеЮВЦјжаЕФ![]() ЃЌЩњГЩЮяжЪЕФСПжЎБШЮЊЕФ1:1ЕФСНжжбЮЃЌвЛжжЮЊ
ЃЌЩњГЩЮяжЪЕФСПжЎБШЮЊЕФ1:1ЕФСНжжбЮЃЌвЛжжЮЊ![]() ЃЌСэвЛжжЮЊ_________________ЁЃ
ЃЌСэвЛжжЮЊ_________________ЁЃ
ЂђЃЎ![]() ЕФКЌСПВтЖЈ
ЕФКЌСПВтЖЈ
ВНжш1ЃКСПШЁ![]() ШмвК
ШмвК![]() ЃЌЯЁЪЭГЩ
ЃЌЯЁЪЭГЩ![]() ЪдбљЃЛСПШЁ
ЪдбљЃЛСПШЁ![]() ЪдбљМгШыЕНзЖаЮЦПжаЃЛ
ЪдбљМгШыЕНзЖаЮЦПжаЃЛ
ВНжш2ЃКЕїНкЪдбљЕФ![]() ЃЌМгШызуСПЕФ
ЃЌМгШызуСПЕФ![]() ОЇЬхЃЌеёЕДКѓЃЌОВжУЦЌПЬЃЛ
ОЇЬхЃЌеёЕДКѓЃЌОВжУЦЌПЬЃЛ
ВНжш3ЃКМгШыжИЪОМСЃЌгУ![]() ШмвКЕЮЖЈжСжеЕуЃЌЯћКФ
ШмвКЕЮЖЈжСжеЕуЃЌЯћКФ![]() ШмвК
ШмвК![]() ЁЃ
ЁЃ
(4)вбжЊЃК![]() ЃЌ
ЃЌ![]() ЃЌд
ЃЌд![]() ШмвКЕФХЈЖШЮЊ_____
ШмвКЕФХЈЖШЮЊ_____![]() ЃЈгУКЌзжФИЕФДњЪ§ЪНБэЪОЃЉЃЌШчЙћЕЮЖЈЫйЖШЙ§Т§ЃЌЛсЪЙМЦЫуГіЕФЪ§жЕ______ЃЈЬюЁАЦЋДѓЁБЁЂЁАЦЋаЁЁБЛђЁАВЛБфЁБЃЉЁЃ
ЃЈгУКЌзжФИЕФДњЪ§ЪНБэЪОЃЉЃЌШчЙћЕЮЖЈЫйЖШЙ§Т§ЃЌЛсЪЙМЦЫуГіЕФЪ§жЕ______ЃЈЬюЁАЦЋДѓЁБЁЂЁАЦЋаЁЁБЛђЁАВЛБфЁБЃЉЁЃ
ЂѓЃЎЩшМЦЪЕбщбщжЄ![]() ЕФбѕЛЏад
ЕФбѕЛЏад
(5)ШЁЪЪСП![]() ЫЎШмвКМгШы
ЫЎШмвКМгШы![]() ШмвКжаЃЌеёЕДЃЌЕУЮоЩЋШмвКЁЃгћМьбщ
ШмвКжаЃЌеёЕДЃЌЕУЮоЩЋШмвКЁЃгћМьбщ![]() ЕФбѕЛЏВњЮяЃЌЛЙашвЊгУЕНЕФЪдМСЪЧ____________ЁЃ
ЕФбѕЛЏВњЮяЃЌЛЙашвЊгУЕНЕФЪдМСЪЧ____________ЁЃ
(6)жЄУї![]() ЕФбѕЛЏадБШ
ЕФбѕЛЏадБШ![]() ЧПЕФЗНАИЪЧ__________________________________ЁЃ
ЧПЕФЗНАИЪЧ__________________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЂёЃЎвбжЊЃКRЃCHЃНCHЃOЃRЁф![]() RЃCH2CHO + RЁфOH
RЃCH2CHO + RЁфOH
ЃЈЬўЛљЯЉЛљУбЃЉ
ЬўЛљЯЉЛљУбAЕФЯрЖдЗжзгжЪСПЃЈMrЃЉЮЊ176ЃЌЗжзгжаЬМЧтдзгЪ§ФПБШЮЊ3ЁУ4 ЁЃгыAЯрЙиЕФЗДгІШчЯТЃК
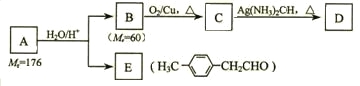
ЧыЛиД№ЯТСаЮЪЬтЃК
ЂХ AЕФЗжзгЪНЮЊ_________________ЁЃ
ЂЦ BЕФУћГЦЪЧ__________ЃЛAЕФНсЙЙМђЪНЮЊ_______________ЁЃ
ЂЧаДГіC Ёњ DЗДгІЕФЛЏбЇЗНГЬЪНЃК_____________________________ЁЃ
ЂШаДГіСНжжЭЌЪБЗћКЯЯТСаЬѕМўЕФEЕФЭЌЗжвьЙЙЬхЕФНсЙЙМђЪНЃК
ЂйЪєгкЗМЯуШЉЃЛ ЂкБНЛЗЩЯгаСНжжВЛЭЌЛЗОГЕФЧтдзгЁЃ
_________________________ЁЂ________________________ЁЃ
ЂђЃЎгЩEзЊЛЏЮЊЖдМзЛљБНввШВЃЈ![]() ЃЉЕФвЛЬѕТЗЯпШчЯТЃК
ЃЉЕФвЛЬѕТЗЯпШчЯТЃК

ЂЩаДГіGЕФНсЙЙМђЪНЃК___________________________ЁЃ
ЂЪаДГіЂй-ЂмВНЗДгІЫљМгЪдМСЁЂЗДгІЬѕМўКЭЂй-ЂлВНЗДгІРраЭЃК____________
ађКХ | ЫљМгЪдМСМАЗДгІЬѕМў | ЗДгІРраЭ |
Ђй | ||
Ђк | ||
Ђл | ||
Ђм | ЁЊЁЊ |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЃЈ1ЃЉМвгУвКЛЏЦјЕФжївЊГЩЗжжЎвЛЪЧЖЁЭщЃЌЕБ10kgЖЁЭщЭъШЋШМЩеВЂЩњГЩЖўбѕЛЏЬМКЭвКЬЌЫЎЪБЃЌЗХГіШШСПЮЊ5ЁС105kJЃЌЪдаДГіБэЪОЖЁЭщШМЩеШШЕФШШЛЏбЇЗНГЬЪНЃК_____________________________________________________
ЃЈ2ЃЉвбжЊЃКCЃЈsЃЉЃЋO2ЃЈgЃЉ===CO2ЃЈgЃЉЃЛІЄH=Ѓ393.5 kJ/molЃЛ2H2ЃЈgЃЉЃЋO2ЃЈgЃЉ===2H2OЃЈgЃЉЃЛІЄH=Ѓ483ЃЎ6 kJ/molЃЌЯжга0ЃЎ2 molЕФЬПЗлКЭЧтЦјзщГЩЕФаќИЁЦјЃЌвђЛьКЯЮядкбѕЦјжаЭъШЋШМЩеЃЌЙВЗХГі63ЃЎ53 kJШШСПЃЌдђЛьКЯЮяжаCгыH2ЕФЮяжЪЕФСПжЎБШЮЊ________________
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com