

| ”÷Cu |
| ”÷Cu |
| ÅØH2SO4 |
| ”÷ |
| ÅØH2SO4 |
| ”÷ |

| ”÷Cu |
| ÅØH2SO4 |
| ”÷ |
| ”÷Cu |
| ÅØH2SO4 |
| ”÷ |


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
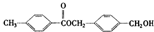 £¬“ÓA³ö·¢£¬æÉ·¢ÉśĶ¼Ź¾ÖŠµÄŅ»ĻµĮŠ·“Ó¦£®ĘäÖŠKµÄ·Ö×ÓŹ½ĪŖC12H14O4£¬LŗĶK»„ĪŖĶ¬·ÖŅģ¹¹Ģ壬FµÄ²śĮææÉ×÷ĪŖŗāĮæŅ»øö¹ś¼ŅŹÆÓĶ»Æ¹¤·¢Õ¹Ė®Ę½µÄ±źÖ¾£®£ØĶ¼ÖŠ[O]±ķŹ¾Ńõ»Æ£©
£¬“ÓA³ö·¢£¬æÉ·¢ÉśĶ¼Ź¾ÖŠµÄŅ»ĻµĮŠ·“Ó¦£®ĘäÖŠKµÄ·Ö×ÓŹ½ĪŖC12H14O4£¬LŗĶK»„ĪŖĶ¬·ÖŅģ¹¹Ģ壬FµÄ²śĮææÉ×÷ĪŖŗāĮæŅ»øö¹ś¼ŅŹÆÓĶ»Æ¹¤·¢Õ¹Ė®Ę½µÄ±źÖ¾£®£ØĶ¼ÖŠ[O]±ķŹ¾Ńõ»Æ£©
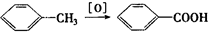









²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(5·Ö)ŅŃÖŖ£ŗ¢ŁAŹĒŹÆÓĶĮŃ½āĘųµÄÖ÷ŅŖ³É·Ż£¬AµÄ²śĮæĶس£ÓĆĄ“ŗāĮæŅ»øö¹ś¼ŅµÄŹÆÓĶ»Æ¹¤Ė®Ę½¢Ś2CH3CHO+O2![]() 2CH3COOH”£ĻÖŅŌAĪŖÖ÷ŅŖŌĮĻŗĻ³ÉE£¬EŹĒŅ»ÖÖÓŠĻćĪ¶µÄÓŠ»śĪļ£¬ĘäŗĻ³ÉĀ·ĻßČēĻĀĶ¼ĖłŹ¾”£
2CH3COOH”£ĻÖŅŌAĪŖÖ÷ŅŖŌĮĻŗĻ³ÉE£¬EŹĒŅ»ÖÖÓŠĻćĪ¶µÄÓŠ»śĪļ£¬ĘäŗĻ³ÉĀ·ĻßČēĻĀĶ¼ĖłŹ¾”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
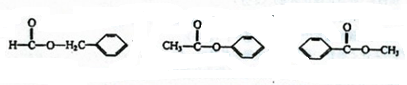
£Ø1£©B”¢E·Ö×ÓÖŠµÄ¹ŁÄÜĶÅĆū³Ę·Ö±šŹĒ ”¢ ”£
£Ø2£©Š“³öĻĀĮŠ·“Ó¦µÄ·“Ó¦ĄąŠĶ£ŗ¢Ü ”£
£Ø3£©Š“³ö·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2015½ģ¹óÖŻŹ”øßŅ»µŚ¶žŃ§ĘŚĘŚÄ©¼ģ²ā»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĶʶĻĢā
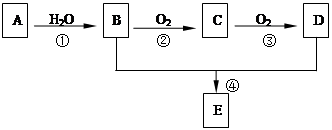
ŅŃÖŖÓŠ»śĪļA”¢B”¢C”¢D”¢E”¢FÓŠŅŌĻĀ×Ŗ»Æ¹ŲĻµ”£AµÄ²śĮæŹĒŗāĮæŅ»øö¹ś¼ŅŹÆÓĶ»Æ¹¤Éś²śĖ®Ę½µÄ±źÖ¾£»DÄÜŹ¹ŹÆČļŹŌŅŗ±äŗģ£»EŹĒ²»ČÜÓŚĖ®ĒŅ¾ßÓŠĖ®¹ūĻćĪ¶µÄĪŽÉ«ŅŗĢ壬Ļą¶Ō·Ö×ÓÖŹĮæŹĒCµÄ2±¶£»FŹĒĖÜĮĻµÄÖ÷ŅŖ³É·ÖÖ®Ņ»£¬³£ÓĆÓŚÖĘŹ³Ę·°ü×°“ü”£½įŗĻĻĀĶ¼¹ŲĻµ»Ų“šĪŹĢā£ŗ

¢Å°“ŅŖĒó»Ų“šĻĀĮŠĪŹĢā£ŗ
¢ŁŠ“³öA”¢EµÄ½į¹¹¼ņŹ½£ŗA ”¢E £»
¢ŚŠ“³öB”¢C”¢D”¢EÖŠ¹ŁÄÜĶŵÄĆū³Ę£ŗB ”¢C”¢ D E”¢ £»
¢ŪŠ“³ö·“Ó¦¢ŚµÄ·“Ó¦·½³ĢŹ½£ŗ
£Ø2£©AÓė±½¶¼ŹĒŹÆÓĶ»Æ¹¤µÄÖŲŅŖ²śĘ·£¬ŌŚŅ»¶ØĢõ¼žĻĀAæÉŅŌ×Ŗ»ÆÉś³É±½£¬°“ŅŖĒó»Ų“šĻĀĮŠĪŹĢā£ŗ
¢Ł±½æÉŅŌ·¢ÉśČ”“ś·“Ó¦£¬Š“³öÓɱ½Öʱøäå±½µÄ»Æѧ·“Ó¦·½³ĢŹ½£ŗ
¢Ś“æ¾»µÄäå±½ŹĒĪŽÉ«ÓĶדŅŗĢ壬ŹµŃéŹŅÖʵƵēÖäå±½Ķس£ŅņČܽāĮĖBr2³ŹŗÖÉ«£¬æÉŅŌ¼ÓČėŹŌ¼Į
³żČ„£¬·“Ó¦·½³ĢŹ½ĪŖ £¬øĆ³żŌÓ²Ł×÷Ėł±ŲŠėµÄÖ÷ŅŖ²£Į§ŅĒĘ÷ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2008-2009ѧğ±±¾©ŹŠĪ÷³ĒĒųøßŅ»£ØĻĀ£©ĘŚÄ©»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
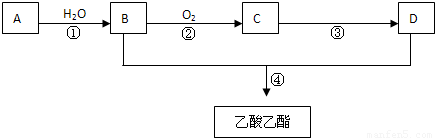
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com