
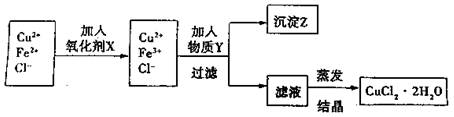
| | �������↑ ʼ����ʱ��pH ʼ����ʱ��pH | �������������ȫʱ��pH |
| Fe3+ | 1.9 | 3.2 |
| Cu2+ | 4.7 | 6.7 |
 ��������ţ�
��������ţ� ��
�� ++H2�����ʵ������ȡCuCl2��Һ����ͬѧ��Ƶ�װ��Ӧ��Ϊ �����ԭ��ء����ء���
++H2�����ʵ������ȡCuCl2��Һ����ͬѧ��Ƶ�װ��Ӧ��Ϊ �����ԭ��ء����ء���
| ���� | FeS | MnS | CuS | PbS | HgS | ZnS |
| Ksp | 6.3��10-18 | 2.5��-13 | 1.3��10-36 | 3.4��10-28 | 6.4��10-53 | 1.6��10-24 |
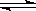 Fe2+��aq��+3OH-��aq����2�֣�
Fe2+��aq��+3OH-��aq����2�֣�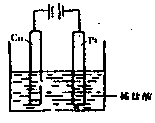 ��3�֣�
��3�֣� ++H2����֪��װ��ֻ���ǵ��أ���Ϊͭ���ᷴӦ�����û�����������Ϊͭʧȥ���ӣ�����ֻ��������������ʺ��������ӵ��ἴ�ɡ�
++H2����֪��װ��ֻ���ǵ��أ���Ϊͭ���ᷴӦ�����û�����������Ϊͭʧȥ���ӣ�����ֻ��������������ʺ��������ӵ��ἴ�ɡ�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A��SO2��H2S��Cl2 | B��SO2��O2��NH3 |
| C��SO2��CO2��O2 | D��HCl��H2S��HI |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A��Ũ�����ͭ��Ӧ�Ʊ�NO2 |
| B��Ũ��ˮ����ʯ�ҷ�Ӧ�Ʊ����� |
| C��п�����ᷴӦ�Ʊ����� |
| D��̼��ƺ����ᷴӦ�Ʊ�������̼ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
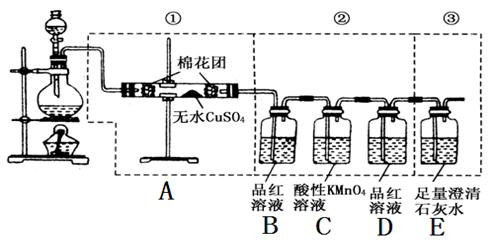
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
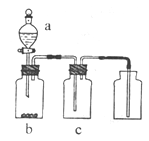
| | ���� | a | b | C |
| A | NO2 | Ũ���� | ͭƬ | NaOH��Һ |
| B | SO2 | Ũ���� | ͭƬ | ����KMnO4��Һ |
| C | CO2 | ϡ���� | Na2CO3���� | Ũ���� |
| D | NH3 | Ũ��ˮ | ��ʯ�� | Ũ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
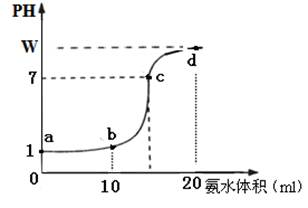
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
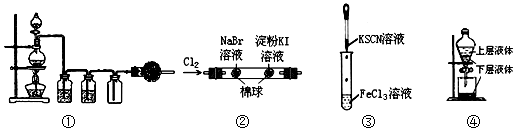
| A���ٿ�����ʵ������MnO2������Ũ���Ṳ�ȣ���ȡ���ռ����������Cl2 |
| B����ʵ��������������ɫ���ұ�������ɫ������֤�������ԣ�Cl2��Br2��I2 |
| C����ʵ���Թ��л�����Ѫ��ɫ���� |
| D����ͼ�ܲ�������ȡ��ˮ�еĵ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com