
��1mol I2 ��g����2 mol H2����ij�ݻ�Ϊ2 L���ܱ������У���һ���¶��·�����Ӧ��I2 ��g��+H2 ��g�� ![]() 2HI��g�����÷�Ӧ����Ӧ���ȣ������ﵽƽ�⡣HI���������
2HI��g�����÷�Ӧ����Ӧ���ȣ������ﵽƽ�⡣HI���������![]() ��HI����ʱ��ı仯��ͼ�����ߣ�����ʾ��
��HI����ʱ��ı仯��ͼ�����ߣ�����ʾ��

��1���ﵽƽ��ʱ��I2 ��g�������ʵ���Ũ��Ϊ_________mol/L��
��2�����ı䷴Ӧ�������ڼ�������![]() ��HI���ı仯��ͼ�����ߣ�����ʾ���������������______________ ������������������ţ�
��HI���ı仯��ͼ�����ߣ�����ʾ���������������______________ ������������������ţ�
�ٺ��������£������¶� �ں��������£������¶�
�ۺ��������£���С��Ӧ��������� �ܺ��������£�����Ӧ���������
�ݺ��º��������£������ʵ�����
��3���������ݻ��ȶ����䣬����һ�ݻ�ҲΪ2 L���ܱ������м���a mol I2 ��g����b mol H2��c mol HI��a��b��c������0����������Ӧ�ﵽƽ��ʱ��HI�����������Ϊ0.60����a��b��c�Ĺ�ϵ��______________��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
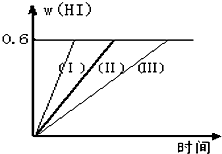 ��1mol I2��g�� ��2mol H2����2L�ܱ������У���һ���¶��·�����Ӧ��I2��g��+H2��g��?2HI��g������H��0������ƽ�⣮HI���������w��HI����ʱ��仯��ͼ���ߣ�����ʾ��
��1mol I2��g�� ��2mol H2����2L�ܱ������У���һ���¶��·�����Ӧ��I2��g��+H2��g��?2HI��g������H��0������ƽ�⣮HI���������w��HI����ʱ��仯��ͼ���ߣ�����ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
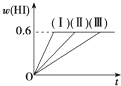 ��1mol I2��g����2mol H2����5L�ܱ������У���һ���¶��·�����Ӧ��I2��g��+H2��g��?2HI��g����H��0�����ﵽƽ�⣮HI���������w��HI����ʱ��仯�����ߣ�����ʾ��
��1mol I2��g����2mol H2����5L�ܱ������У���һ���¶��·�����Ӧ��I2��g��+H2��g��?2HI��g����H��0�����ﵽƽ�⣮HI���������w��HI����ʱ��仯�����ߣ�����ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| H | + 4 |
| O | - 2 |
| H | + 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�����ʡ�����и����ڶ��ε��п��Ի�ѧ�Ծ� ���ͣ������
��18�֣���1��ijһ��Ӧ��ϵ���з�Ӧ��������ﹲ5�����ʣ�S��H2S��HNO3��NO��H2O��
д���÷�Ӧ�Ļ�ѧ����ʽ����ƽ_________________________________������Ӧ������
ת����0��3mol���ӣ������������������____________g��
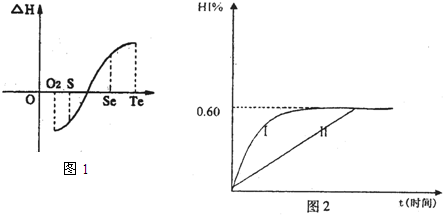
��2��ͬһ���ʳ���̬����ֵ���Һ̬����ֵ��֮����̬����ֵ��С����ͬ��ͬѹ��һ����ѧ��Ӧ�����������������ڷ�Ӧ�����������Ϳ��Դ�����Ϊ�÷�Ӧ���ر�Ϊ0��ij��ѧ��ȤС�飬ר���о�������Ԫ�ؼ���ijЩ������IJ������ʡ������������£�

a���ڣ�Te��Ϊ���壬H2TeΪ���壬Te��H2����ֱ�ӻ�������H2Te
b�������ʵ�������������������H2��Ӧ���ʱ������ͼl��ʾ��
��ش��������⣺
H2���ϵķ�Ӧ______________��������ų��������ա�����������Ŀ������Ϣ�������ΪʲôTe��H2����ֱ�ӻ���_________________________________________.
��3���ڸ��ӵķ�Ӧ�У�Ҫ���Ƿ�Ӧ���Ⱥ�˳����֪ ��
�� ��2H2O
��2H2O Al��OH��3�� ��NH3��H2O�����е����ʵ�����
Al��OH��3�� ��NH3��H2O�����е����ʵ����� ��Al3����H�������Һ�У������μ�NaOH��Һ��ֱ�������������Ͻ��裬���η������������ӷ�Ӧ�����У�
��Al3����H�������Һ�У������μ�NaOH��Һ��ֱ�������������Ͻ��裬���η������������ӷ�Ӧ�����У�
�ڶ������ӷ�Ӧ�����ӷ���ʽ��__________________________________.
������ӷ�Ӧ�����ӷ���ʽ��________________________________.
��4����1mol I2��g����2mol H2��g������ij2L�ܱ������У���һ���¶��·�����Ӧ��H2��g����I2��g�� 2HI��g������H<0�� ����ƽ�⡣HI���������HI����ʱ�ʱ仯������ͼ2��ʾ��
2HI��g������H<0�� ����ƽ�⡣HI���������HI����ʱ�ʱ仯������ͼ2��ʾ��

�ٴﵽƽ��ʱ��I2��g�������ʵ���Ũ��Ϊ________mol��L.
�ڱ��ּ���ķ�Ӧ������ʵ������䣬���ı䷴Ӧ��������ijһ������HI���ı仯������l��ʾ��������������ǣ�д�����еĿ����ԣ�___________________________________________________________�����������£�ƽ�ⳣ��Kֵ____________�����������С���������䡱���ܱ��Ҳ���ܱ�С����
���������¶Ȳ��䣬����һ��ͬ��2L�ܱ������м���1molH2��g����2molHI��g����������Ӧ�ﵽƽ��ʱ��H2���������Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
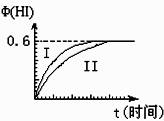
��1mol I2��g����2mol H2��g������ij2L�ܱ������У���һ���¶��·�����Ӧ��
I2��g��+H2��g��![]() 2HI��g��; ��H<0�����ﵽƽ�⡣HI�������������HI����ʱ��仯��ͼ���ߣ�II����ʾ��
2HI��g��; ��H<0�����ﵽƽ�⡣HI�������������HI����ʱ��仯��ͼ���ߣ�II����ʾ��
��1����ƽ��ʱ��I2��g�������ʵ���Ũ��Ϊ mol?L-1��
��2�����ı䷴Ӧ��������ij�����¦���HI���ı仯������
��I����ʾ��������������� ������������������ţ���
�ٺ��������£������¶�
�ں��������£������¶�
�ۺ��������£���С��Ӧ�������
�ܺ��������£�����Ӧ�������
�ݺ��¡����������£������ʵ�����
��3���������¶Ȳ��䣬����һ��ͬ��2L�ܱ������м���a mol I2��g����b mol H2��g����c mol HI��g����a��b��c������0����������Ӧ��ƽ��ʱ��HI�����������Ϊ0.60,��a��b��c�Ĺ�ϵΪ ����һ����a��b��c�Ĵ���ʽ��ʾ����
��4������ʱ��0.01 mol HI��������ˮ���100 ml��Һ�������Һ����ˮ��������������ӵ����ʵ���Ũ��Ϊ mol?L-1 ���������¶ȸ���Һ��pHֵ�� ��������С�䣩
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com