
��1�����и�����������������̼ԭ�ӵ���______________��
A��BrCH2CHOHCH2OH���� B��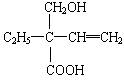
C��CH3CHOHCOOH�������� D��CH3COCH2CH3
��2��Ҫʹ��1���з��ӵ�������ʧ���ֱ�ɲ�ȡ�Ĵ�ʩ���Բ��ı�̼ԭ����Ϊǰ�ᣬ��ֻһ�����ǣ����л���Ӧ���ͣ����ֿ���ʱ��ֻ��һ�ּ��ɡ�û�ж�ӳ�칹����IJ����
A��____________________B��__________________
C��____________________D��__________________
��3����д��ʹ��1����A��D������ʧ������һ���л���Ӧ����ʽ��
___________________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ũ���� |
| �� |
| Ũ���� |
| �� |
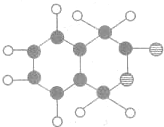
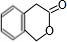

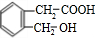
| ���� |
| �� |
 +��n-1��H2O
+��n-1��H2O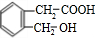
| ���� |
| �� |
 +��n-1��H2O
+��n-1��H2O ��
��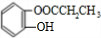 ��
��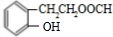
 ��
��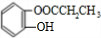 ��
��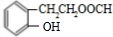
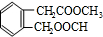
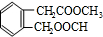
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����л�����һ��������Ԫ�� | B�����л��������C��H��O��ԭ�Ӹ�����Ϊ1��2��3 | C��������C��H��ԭ�Ӹ�����Ϊ1��2 | D������ٲ�ø��л�����Һ̬�����ܶȼ���ȷ�����ķ���ʽ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
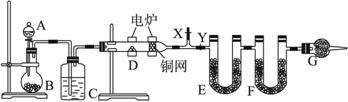
����������⣺
(1)��ƿB��Ϊ��ɫ���壬�����з�����Ӧ�Ļ�ѧ����ʽΪ_________________________��
(2)ͭ����������________________________��
(3)F�е��Լ������____________��
(4)װ��G��������________________________��
(5)�ڿ�ʼ����D֮ǰ���ȴ���X���رռ���Y��ͨһ��ʱ���������ٹرռ���X������Y��Ȼ��ʼ���ȣ�����������Ŀ����______________________________________��
(6)��ȡ1.80 gij�л������(�������ĺ���������)�������������ⶨ��E������10.8 g��F������2.64 g������л�������ʽΪ_________________________�����Ҫȷ���л���ķ���ʽ������ⶨ��������___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�����ʽṹ�����ʡ�ģ��ѡ����
1.�������ʲ����ý��������۽��͵���
A.������ B.������ C.��չ�� D.��ʴ��
2.����˵���������
A.O3��SO2�Ľṹ����������
B.��۲�����ˮ��������CS2��
C.Be��OH��2��������������
D.����ͬ�����£�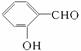 �ķе����
�ķе����![]()
3.��������ˮʱ����NH3��H2O��������á�������ʾ������γ�NH3��H2O���ӣ����ݰ�ˮ�����ʿ���֪NH3��H2O�ĽṹʽΪ
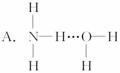


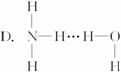
���л���ѧ������ģ��ѡ����
4.��֪ij�л���A�ķ���ʽC2H6O�ĺ�����ͺ˴Ź�����������ͼ��ʾ������˵���д������
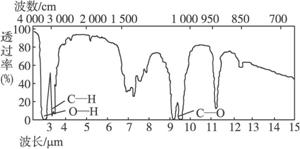
δ֪��A�ĺ������
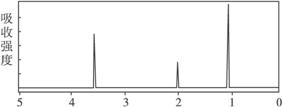
δ֪��A�ĺ˴Ź�������
A.�ɺ������֪�����л��������ٺ������ֲ�ͬ�Ļ�ѧ��
B.�ɺ˴Ź�������֪�����л�������������ֲ�ͬ����ԭ���Ҹ�����Ϊ1��2��3
C.������˴Ź�������֪������еĸ�������ԭ������
D.��A�Ľṹ��ʽΪCH3��O��CH3
5.�ڰ�˾ƥ�ֵĽṹ��ʽ����ʽ���Т٢ڢۢܢݢֱ�����������еIJ�ͬ�ļ�������˾ƥ��������NaOH��Һ����ʱ��������Ӧʱ�ϼ���λ����
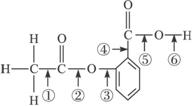
A.�٢� B.�ڢ� C.�ۢ� D.�ڢ�
6.�ٰ조���İ��ˡ���һ����Ҫ���־��ǽ�ֹ�˶�Ա�����˷ܼ�����һ���˷ܼ��Ľṹ��ʽ����ʽ�������йظ����ʵ�˵����ȷ����
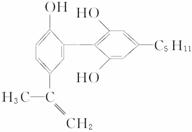
A.�÷���������̼ԭ�ӿ����ȶ��ع�����һ��ƽ����
B.1 mol��������Ũ��ˮ��H2��Ӧʱ���������Br2��H2�����ʵ����ֱ�Ϊ4 mol��7 mol
C.��FeCl3��Һ����ɫ����Ϊ�������뱽������ͬϵ��
D.��������KMnO4��Һ���۲쵽��ɫ��ȥ����֤�������д���˫��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com