

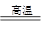 Fe3O4 + 4H2
Fe3O4 + 4H2


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
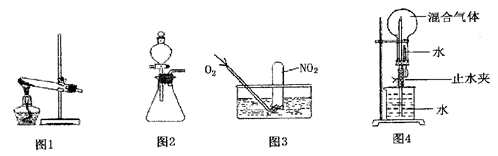
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

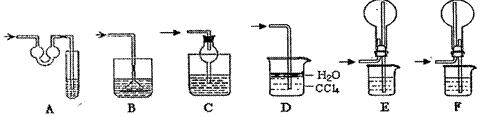
| ��� | þ���Ͻ����� | �����ܵ�һ�ζ��� | �����ܵڶ��ζ��� |
| �� | 1.0g | 10.0mL | 346.3mL |
| �� | 1.0g | 10.0mL | 335.0mL |
| �� | 1.0g | 10.0mL | 345.7mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
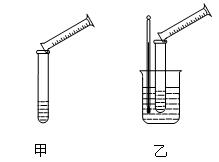
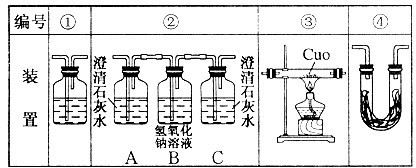
| ʵ�� ��� | �¶�] | Na2S2O3��Һ | H2SO4 | ����H2O����� | ���ֳ�������ʱ�� | ||
| ��� | Ũ�� | ��� | Ũ�� | ||||
| �� | 0�� | 5mL | 0.1mol��L��1 | 10mL | 0.1mol��L��1 | 5mL | 8s |
| �� | 0�� | 5mL | 0.1mol��L��1 | 5mL | 0.1mol��L��1 | 10mL | 12s |
| �� | 30�� | 5mL | 0.1mol��L��1 | 5mL | 0.1mol��L��1 | 10mL | 4s |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
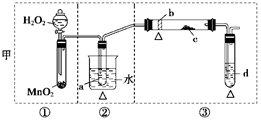
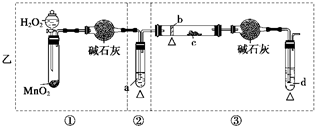
 չ���о������и��л�ѧ�̲��жԡ����ȷ�Ӧ��������������������������Ӧ�ų��������ȣ�������ҫ�۵Ĺ�â������ֽ©�����²����մ���������������ɳ�С������ġ���ѧ
չ���о������и��л�ѧ�̲��жԡ����ȷ�Ӧ��������������������������Ӧ�ų��������ȣ�������ҫ�۵Ĺ�â������ֽ©�����²����մ���������������ɳ�С������ġ���ѧ �ֲᡷ֪��Al��Al2O3��Fe��Fe2O3�۵㡢�е��������£�
�ֲᡷ֪��Al��Al2O3��Fe��Fe2O3�۵㡢�е��������£�| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1462 |
| �е�/�� | 2467 | 2980 | 2750 | - |
 (�����)��
(�����)��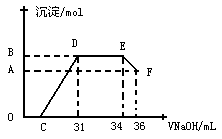
 ��Ӧ�ij��������ʵ���Ϊ________mol��C���Ӧ������������Һ�����Ϊ___________mL
��Ӧ�ij��������ʵ���Ϊ________mol��C���Ӧ������������Һ�����Ϊ___________mL�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com