
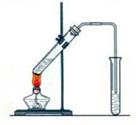
 ��ҵ����ϵ�д�
��ҵ����ϵ�д� ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д� �����ܾ�ϵ�д�
�����ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ | B�����Ҵ������ |
| C����������̼������Һϴ�Ӻ��Һ | D����������������Һϴ�Ӻ��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����л��������������ڡ��������ࡱ�����ࡱ���������ࡱ |
| B�����������ᷴӦ��Ҳ�����������Һ��Ӧ |
| C�����ᶡ��������COOC4H9����3�ֲ�ͬ�ṹ |
| D�����ķ���ʽC11H11NO2 |
�鿴�𰸺ͽ���>>
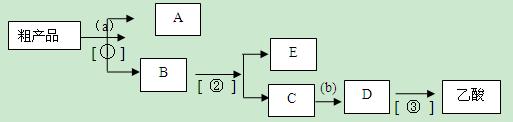
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����


 C�ڵõ�����ϩ��Ʒ��
C�ڵõ�����ϩ��Ʒ��
 �˵�������е������� ��
�˵�������е������� ��
 ��
��
�鿴�𰸺ͽ���>>
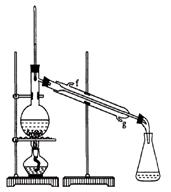
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
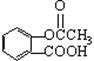 ����˵���������
����˵���������| A����˾ƥ�������������л��� | B����˾ƥ���н�����ʹ������ |
| C�����ð�˾ƥ�ֿ��ܳ��ֲ�����Ӧ | |
| D����˾ƥ�ֲ���ˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
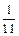 ������������Ԫ�ص����������ǣ� ��
������������Ԫ�ص����������ǣ� ��A��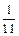 | B��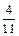 | C��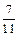 | D��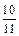 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ����� | �Լ� | ���뷽�� |
| A | �������ӣ�[ | ��ˮ | ��Һ |
| B | �Ҵ���ˮ�� | �� | ���� |
| C | �屽���壩 | ����������Һ | ��Һ |
| D | �������������ᣩ[] | ����̼������Һ | ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com