
| Ũ���� |
| �� |
| Ũ���� |
| �� |
| ���� |
| ���� |
| Ũ���� |
| �� |
| Ũ���� |
| �� |
| ���� |
| ���� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2013?������һģ���£�N2H4���Ͱ��ǵ������ֳ���������ڿ�ѧ�������������й㷺Ӧ�ã��밴Ҫ��ش��������⣺
��2013?������һģ���£�N2H4���Ͱ��ǵ������ֳ���������ڿ�ѧ�������������й㷺Ӧ�ã��밴Ҫ��ش��������⣺

| ||
| ||
| T/�� | 165 | 175 | 185 | 195 |
| K | 111.9 | 74.1 | 50.6 | 34.8 |
| n(NH3) |
| n(CO2) |
| n��N2�� | n��H2�� | n��NH3�� | |
| �� | 1mol | 3mol | 0mol |
| �� | 0.5mol | 1.5mol | 1mol |
| �� | 0mol | 0mol | 4mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
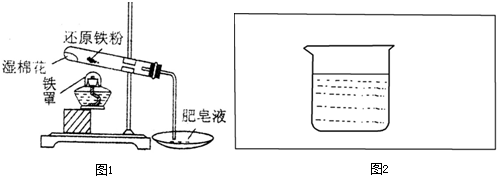
 ����ʾ�������һ��ԭ���װ�ã�����֤�е����������������ͼ2�����ڲ���ȫװ�ü�ͼ��Ҫ���ע��������������Ϻ͵������Һ���ƣ���
����ʾ�������һ��ԭ���װ�ã�����֤�е����������������ͼ2�����ڲ���ȫװ�ü�ͼ��Ҫ���ע��������������Ϻ͵������Һ���ƣ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016���Ĵ�ʡ�����и�һ��ѧ��12���¿���ѧ�Ծ��������棩 ���ͣ������
�����ʵķ�����ᴿ�ж��ַ��������ʷ��롢�ᴿ����������ڿ�ѧ�о���ҵ������ռ��ʮ����Ҫ�ĵ�λ����ҵ��ұ������ԭ����������(��Ҫ�ɷ���Al2O3������ΪFe2O3��SiO2�ȣ���֪SiO2�Dz�����ˮ�����������Fe2O3�Dz�����ˮ�ļ���������)��ij�о�С����Ƶ��ᴿAl2O3�ķ������£�
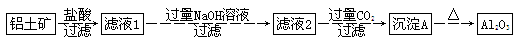
��1��д������A�Ļ�ѧʽ��___________________________________________________��
��2�����������NaOH��Һ�����˺����Һ�к��е�������________________________��
��3��д���ɳ���A�D��Al2O3�Ļ�ѧ����ʽ��________________________��ͨ�����CO2���ɳ���Aʱ��Ӧ�����ӷ���ʽΪ_______________________________________________��
����Ҫ��ش��������⣺
A��B��C��D��E�dz�����������ʣ�������ת����ϵ (��ȥ��������Ʒ����
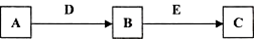
��1����AΪ����ɫ���嵥�ʣ�D��EΪ�����г��������ֽ���������E��һ�ֺ�ɫ���������ʡ�
�� д����B����Һ�м�������D�����ӷ�Ӧ����ʽ_____________________________________��
��������õ���Һ�м���NaOH��Һ�����ڿ����з��õ������ǣ�_________________________��д�������ڿ����з��õĻ�ѧ��Ӧ����ʽ��__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �£�N2H4���Ͱ��ǵ������ֳ���������ڿ�ѧ�������������й㷺Ӧ�ã��밴Ҫ��ش��������⣺
�£�N2H4���Ͱ��ǵ������ֳ���������ڿ�ѧ�������������й㷺Ӧ�ã��밴Ҫ��ش��������⣺
��1��N2H4��Nԭ�Ӻ��������ﵽ8�����ȶ��ṹ��д��N2H4�Ľṹʽ��______��
��2��ʵ���������ֹ�����ȡNH3�ķ�Ӧ��ѧ����ʽΪ______��
��3��NH3��NaClO��Ӧ�ɵõ��£�N2H4�����÷�Ӧ�Ļ�ѧ����ʽΪ______��
��4����һ����ȼ�ϵ����һ�ּ��Ի�����أ��õ�طŵ�ʱ�������ķ�ӦʽΪ______��
��5����ҵ�������ص�ԭ������NH3��CO2Ϊԭ�Ϻϳ�����[CO��NH2��2]��Ӧ�Ļ�ѧ����ʽΪ2NH3��g��+CO2��g��?CO��NH2��2��l��+H2O��l�����÷�Ӧ��ƽ�ⳣ�����¶ȹ�ϵ���£�
| T/�� | 165 | 175 | 185 | 195 |
| K | 111.9 | 74.1 | 50.6 | 34.8 |
���ʱ��H______0�����������������=������
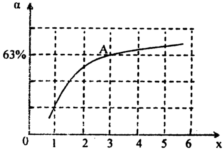
����һ���¶Ⱥ�ѹǿ�£���ԭ�����е�NH3��CO2�����ʵ���֮�ȣ���̼�ȣ�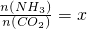 ����ͼ�ǰ�̼�ȣ�x����CO2ƽ��ת���ʣ������Ĺ�ϵ��������x����������ԭ����______��ͼ��A�㴦��NH3��ƽ��ת����Ϊ______��
����ͼ�ǰ�̼�ȣ�x����CO2ƽ��ת���ʣ������Ĺ�ϵ��������x����������ԭ����______��ͼ��A�㴦��NH3��ƽ��ת����Ϊ______��
��6���ں��º����ܱ������а��ռס��ҡ������ַ�ʽ�ֱ�Ͷ�ϣ�������Ӧ��N2��g��+3H2��g��?2NH3��g������ü�������H2��ƽ��ת����Ϊ40%��
| n��N2�� | n��H2�� | n��NH3�� | |
| �� | 1mol | 3mol | 0mol |
| �� | 0.5mol | 1.5mol | 1mol |
| �� | 0mol | 0mol | 4mol |
���ж��������з�Ӧ���еķ�����______�������������ƶ���
�ڴ�ƽ��ʱ���ס��ҡ�����������NH3�����������С˳��Ϊ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com