

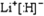
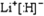
| 0.1mol |
| 0.1L |
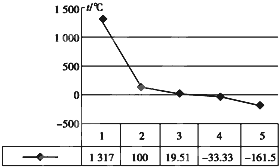
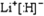 ��
��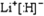 ��
��

 ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д� ���������ν�ϵ�д�
���������ν�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �±���Ԫ�����ڱ���ǰ�����ڣ�
�±���Ԫ�����ڱ���ǰ�����ڣ�| ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 | |
| һ | A | |||||||
| �� | B | C | D | E | F | |||
| �� | G | H | I | J |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �±���Ԫ�����ڱ���ǰ�����ڣ�
�±���Ԫ�����ڱ���ǰ�����ڣ�| ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 | |
| һ | A | |||||||
| �� | B | C | D | E | F | |||
| �� | G | H | I | J | K |
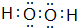
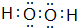
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�




�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015���Ĵ�ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
�±���Ԫ�����ڱ���ǰ�����ڣ���Ա���A��G����Ԫ����գ�
��
���� ��A ��A ��A ��A ��A ��A ��A 0
1 A
2 B C D
3 E F G
��1��A��B����Ԫ�������Է���������С�Ļ��������ӵĿռ�ṹΪ ��
��2������Ԫ���е�����������Ӧˮ�����������ǿ���� ���û�ѧʽ��ʾ����ͬ����������ǿ���� ���û��������� ����ѡ�����ӻۻ�������û�����ĵ���ʽΪ ��������ѧ������ �� ��
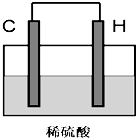
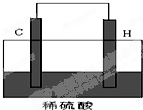
��3����A��D�ĵ��ʿ����Ƴ�ȼ�ϵ�أ������װ��E������������ˮ�����Ũ��Һ���������Һ���ö�Ľ������Ե缫��������Ũ��Һ�У���X��ͨ��D�ĵ��ʣ�Y��ͨ��A�ĵ��ʣ���Y���Ǹõ�ص� ����ѡ���������߸�������X���ĵ缫��Ӧʽ�� ���������ת��1 mol ����ʱ������A���ʵ����Ϊ L���������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com