�⣺��1����̼��識��ȷֽ����������ˮ�Ͷ�����̼����ѧ����ʽΪ����NH
4��
2CO
3
2NH
3��+CO
2��+H
2O���ʴ�Ϊ����NH
4��
2CO
3
2NH
3��+CO
2��+H
2O��
�ڽ���������ˮ�γɰ�ˮ�����ɵİ�����ͨ����ʯ�ҳ�ȥ������̼����ͨ��ˮ�м��ɣ��ʴ�Ϊ��edf��
��2����N
2��g��+3H
2��g��?2NH
3��g����H=-92.4kJ?mol
-1 ��2H
2��g��+O
2��g��=2H
2O��l����H=-572kJ?mol
-1���ݸ�˹���ɣ��ɢڡ�3-�١�2�ã�4NH
3��g��+3O
2��g��=2N
2��g��+6H
2O��l����H=-1531.2 kJ?mol
-1�ʴ�Ϊ��4NH
3��g��+3O
2��g��=2N
2��g��+6H
2O��l����H=-1531.2 kJ?mol
-1��
��3���ٸ÷�Ӧ��ƽ�ⳣ������ʽK=
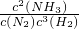
���ʴ�Ϊ��
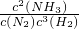
��
����μӷ�Ӧ�ĵ��������ʵ���xmol��
N
2 +3H
2 
2NH
3��ʼ��mol����1 3 0
�仯��mol����x 3x 2x
ƽ�⣨mol����1-x 3-3x 2x
5min��ﵽƽ�⣬ƽ��ʱ�����������Ϊ
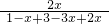
��100%=25%���⣺x=0.4��
N
2�ķ�Ӧ����v��N
2��=
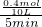
=0.008mol/��L�qmin�����ʴ�Ϊ��0.008mol/��L�qmin����
��H
2��ת����Ϊ
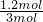
��100%=40%���ʴ�Ϊ��40%��
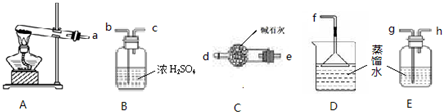
��4���ټ��ȵ�����Ʒ�����������壬����һ��������ʹʪ��ĺ�ɫʯ����ֽ������������Ϊ������˵������笠����ӣ���һ��������ʹ����ʯ��ˮ����ǣ�˵��̼������ӻ�̼��������ӣ���ȡ�����õ�����Ʒ����ˮ������������BaCl
2��Һ��û�����Ա仯��˵��û��̼��������ӣ�˵�����к���笠����Ӻ�̼������ӣ����Ե��ʵ���Ҫ�ɷ�NH
4HCO
3����ѡ��C��
��������1���ٸ���̼��識��ȷֽ����������ˮ�Ͷ�����̼��
�ڸ�����ɿ�֪����������ˮ�γɰ�ˮ�����ɵİ�����ͨ����ʯ�ҳ�ȥ������̼����ͨ��ˮ�м��ɣ�
��2�����ݸ�˹���������
��3���ٻ�ѧƽ�ⳣ����ƽ��ʱ��������Ũ�ȵĻ�ѧ���������ݵij˻����Ը���Ӧ��Ũ�ȵĻ�ѧ���������ݵij˻����õı�ֵ��
����μӷ�Ӧ�ĵ��������ʵ������ٸ������η�������Ե����ʵ������ٸ�������������δ֪����Ȼ�����v=

���㷴Ӧ���ʣ�
�۸���ת���ʵĸ�����м��㣻
��4���ټ��ȵ�����Ʒ�����������壬����һ��������ʹʪ��ĺ�ɫʯ����ֽ������������Ϊ������˵������笠����ӣ���һ��������ʹ����ʯ��ˮ����ǣ�˵��̼������ӻ�̼��������ӣ���ȡ�����õ�����Ʒ����ˮ������������BaCl
2��Һ��û�����Ա仯��˵��û��̼��������ӣ��ɴ��Ƶ������ʵ���Ҫ�ɷ֣�
�����������ǵ��ۺ��⣬�ѶȲ����漰֪ʶ��϶࣬ע���Ӧ֪ʶ�Ļ��ۣ�


 2NH3��5min��ﵽƽ�⣬ƽ��ʱ�����������Ϊ25%��
2NH3��5min��ﵽƽ�⣬ƽ��ʱ�����������Ϊ25%�� 2NH3��+CO2��+H2O���ʴ�Ϊ����NH4��2CO3
2NH3��+CO2��+H2O���ʴ�Ϊ����NH4��2CO3 2NH3��+CO2��+H2O��
2NH3��+CO2��+H2O��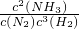 ���ʴ�Ϊ��
���ʴ�Ϊ��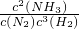 ��
�� 2NH3
2NH3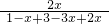 ��100%=25%���⣺x=0.4��
��100%=25%���⣺x=0.4��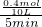 =0.008mol/��L�qmin�����ʴ�Ϊ��0.008mol/��L�qmin����
=0.008mol/��L�qmin�����ʴ�Ϊ��0.008mol/��L�qmin���� 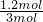 ��100%=40%���ʴ�Ϊ��40%��
��100%=40%���ʴ�Ϊ��40%�� ���㷴Ӧ���ʣ�
���㷴Ӧ���ʣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�