
��֪��CO(g)��H2O(g)CO2(g)��H2(g)����H����41 kJ
��mol��1����ͬ�¶��£����ݻ���ͬ�����������ܱ������У�����һ�����ķ�Ӧ�����Ӧ������������£�
| ������� | ��ʼʱ���������ʵ���/mol | ��ƽ�������ϵ�����ı仯 | |||
| CO | H2O | CO2 | H2 | ||
| �� | 1 | 4 | 0 | 0 | �ų�������32.8 kJ |
| �� | 0 | 0 | 1 | 4 | �����仯��Q |
����˵���У�����ȷ���� (����)��
A���������з�Ӧ�ﵽƽ��ʱ��CO��ת����Ϊ80%
B����������CO��ת���ʵ�����������CO2��ת����
C��ƽ��ʱ����������CO2��Ũ�����
D����������CO��Ӧ���ʵ���H2O�ķ�Ӧ����
������A�1 mol CO��ȫ��Ӧʱ�ų�������Ϊ41 kJ���������з�Ӧ�ų�������Ϊ32.8 kJ������ʵ�ʷ�Ӧ��CO�����ʵ���Ϊ0.8 mol������CO2Ϊ
0.8 mol���������з�Ӧ��ƽ��ʱ��CO��ת����Ϊ80%����ȷ��B��������������ݿɼ������ѧƽ�ⳣ��Ϊ1�����ݻ�ѧƽ�ⳣ���ɼ���ƽ��ʱ��������CO2�����ʵ���Ϊ0.2 mol���Ӷ������CO2��ת����Ϊ80%����ȷ��D���Ӧ����֮�ȵ������ʵĻ�ѧ������֮�ȣ�����CO�ķ�Ӧ���ʵ���H2O�ķ�Ӧ���ʣ���ȷ��C���A��B���������֪ƽ��ʱ����������CO2��Ũ�Ȳ���ȣ���>�ڣ�C����
�𰸡�C



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ݱ�����������M�Է��ѻ�ù���нϺõ��־����ԣ���ϳ�·������ͼ��ʾ��
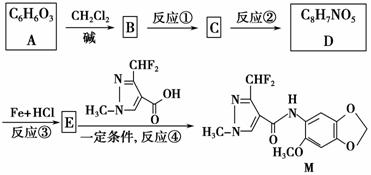

���������գ�
(1)д����Ӧ���ͣ�
��Ӧ��________����Ӧ��________��
(2)д���ṹ��ʽ��
A_____________________________________________________________��
E__________________________________________________________��
(3)д����Ӧ�ڵĻ�ѧ����ʽ��_______________________________________
_________________________________________________________________��
(4)B�ĺ������ṹ��ͬ���칹���У���һ���ܷ�������ˮ�⣬д����������ͬ���칹���еĹ�����(���ǻ�����)���Լ������ֵ�����
�Լ�(��̪����)��___________________________________________��
����_____________________________________________________��
(5)д������C�ĺ������ṹ��ֻ��4�ֲ�ͬ��ѧ������ԭ�ӵ�ͬ���칹��Ľṹ��ʽ��
_________________________________________________________
_________________________________________________________��
(6)��Ӧ�١���Ӧ�ڵ��Ⱥ�����ܵߵ��������ԭ��_________________
____________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������һ�ֳ�����θҩ������Ч�ɷֵĽṹ��ʽ������ͼ��ʾ�����ڸ����ʵ�����˵������ȷ����(����)
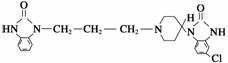
A�������ʵķ���ʽΪC22H24ClN5O2
B�������ʾ��м��ԣ������ᷴӦ
C�������ʲ��ܷ���ˮ�ⷴӦ
D���������ܷ���ȡ����Ӧ�ͼӳɷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�ֽ�����ʹҩ�Ľṹ��ʽΪ ��������ȫˮ��ʱ���ɵõ��IJ�����(����)
��������ȫˮ��ʱ���ɵõ��IJ�����(����)
A��2�� B��3�� C��4�� D��5��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ݻ��ɱ���ܱ������У�1 mol N2��4 mol H2��һ�������·�����Ӧ���ﵽƽ��ʱ��H2��ת����Ϊ25%����ƽ��ʱ�ĵ�������������ӽ��� (����)��
A��5%�� B��10%����
C��15%�� D��20%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��100 ��ʱ����0.100 mol N2O4�������1 L���ݳ�յ��ܱ������У���һ��ʱ��Ը����������ʵ�Ũ�Ƚ��з����õ�������ݣ�
| ʱ��(s) | 0 | 20 | 40 | 60 | 80 |
| c(N2O4)/mol��L��1 | 0.100 | c1 | 0.050 | c3 | c4 |
| c(NO2)/mol��L��1 | 0.000 | 0.060 | c2 | 0.120 | 0.120 |
(1)�÷�Ӧ��ƽ�ⳣ������ʽΪ________���ӱ��з�����
c1________c2��c3________c4(�>������<������)��
(2)�����������£��ӷ�Ӧ��ʼֱ���ﵽ��ѧƽ��ʱ��N2O4��ƽ����Ӧ����Ϊ________mol��L��1��s��1��
(3)��ƽ������������ĸı��ʹNO2����Ũ���������________(����ĸ���)��
A�������������ݻ��� B���ٳ���һ������N2O4
C�������һ������NO2�� D���ٳ���һ������He
(4)������ͬ�����£���ʼʱֻ����0.080 mol NO2���壬��ﵽƽ��ʱNO2�����ת����Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ܱ������н��еĿ��淴Ӧ��aA(g)��bB(g)cC(g)�ڲ�ͬ�¶�(T1��T2)��ѹǿ(p1��p2)�£����������B����������w(B)�뷴Ӧʱ��(t)�Ĺ�ϵ��ͼ��ʾ�������ж���ȷ���� (����)��

A��T1<T2��p1<p2��a��b>c������ӦΪ���ȷ�Ӧ
B��T1>T2��p1<p2��a��b<c������ӦΪ���ȷ�Ӧ
C��T1<T2��p1>p2��a��b<c������ӦΪ���ȷ�Ӧ
D��T1>T2��p1>p2��a��b>c������ӦΪ���ȷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͼ�����о���ѧ��Ӧ���ʱ��һ�ֳ��÷�����

ͼ1
(1)��֪��ѧ��ӦA2(g)��B2(g)===2AB(g)�������仯������ͼ1��ʾ���ж�������������ȷ����________��
A��ÿ����2 mol ABʱ���� b kJ ����
B���÷�Ӧ�Ȧ�H����(a��b) kJ��mol��1
C���÷�Ӧ�з�Ӧ��������������������������
D������1 mol A��A��1 mol B��B��ʱ�ų�a kJ����
(2)���������õ������£�NH ����������Ӧ��������NO
����������Ӧ��������NO ��������Ӧ�������仯��ͼ2��ʾ��
��������Ӧ�������仯��ͼ2��ʾ��
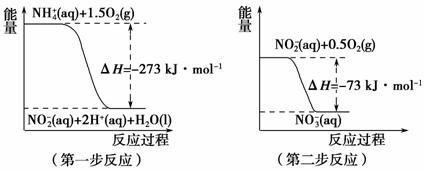
ͼ2
1 mol NH (aq)ȫ��������NO
(aq)ȫ��������NO (aq)���Ȼ�ѧ����ʽ��____________________________________________��
(aq)���Ȼ�ѧ����ʽ��____________________________________________��
(3)ͼ3�б�ʾ����Ԫ���������������������⻯��ʱ���ʱ����ݣ������ʱ����ݿ�ȷ��a��b��c��d�ֱ��������Ԫ�ء�
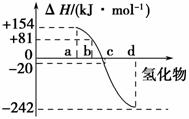
ͼ3
�ٷǽ���Ԫ���⻯����ȶ������⻯�������Ȧ�H�Ĺ�ϵΪ_______________________________________________________________��
��д�������ⷢ���ֽⷴӦ���Ȼ�ѧ����ʽ____________________________
_______________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij����С���������ͼ��ʾװ�ã����ڻ������������ڵ���ǿ�����е������������л������NaCl��Һ�����ʵ��(��ʱ����ֹˮ��a���ر�ֹˮ��b)�����ڴ��ģ�ʵ�鲢δ�ﵽԤ��Ŀ�ģ���Ҳ���������˸��˵�������������Ƿ������ش���������(ͼ�������ӽ���Ĥֻ���������Ӻ�ˮ����ͨ��)��
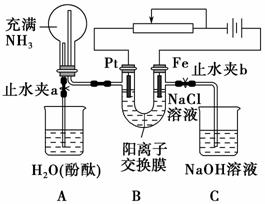
(1)д��Bװ���еĵ缫��Ӧʽ��
Pt��_________________________________________________________��
Fe��____________________________________________________________��
(2)д���۲쵽��Aװ���е�����
��_________________________________________________________��
��__________________________________________________________��
��___________________________________________________________��
(3)���۲쵽Aװ���е���������ǹر�ֹˮ��a����ֹˮ��b���ٹ۲�Cװ�á���������˵�����ɣ�����������д���йط�Ӧ����ʽ________________________________________________________________
_______________________________________________________________��
(4)����ﵽ���NaCl��Һ��Ŀ�ģ�Ӧ��θĽ�װ�ã������������________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com