
��1����δ��HCl��Һ��ϴ��ʽ�ζ��ܾ�װҺ���еζ���
��δ��NaOH����Һ��ϴ��ʽ�ζ��ܾͽ���ȡҺ��
����NaOH����Һ��ϴ��ƿ��
��װ��HCl��Һ�ζ��ܼ������δ�ų��ͽ��еζ���
��װ��NaOH����Һ�ζ��ܼ������δ�ų��ͽ���ȡҺ��
����ʽ�ζ��ܿ�ʼǰ���Ӷ������ζ�����ʱ���Ӷ�����
������ƿȷȡNaOH����Һʱ����ƿ������������ˮ��
��ҡ����ƿʱ��Һ�彦����
��2����HCl��Һ��Ũ����ϡһ��ã�����Ũһ��ã�Ϊʲô��
 ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д� 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
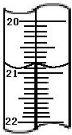 �ӵ�ʳ������ʳ�������ӵ���أ���ʳ�κ���������I���㣩����20��50mg/kg�������������Σ�������Լ��ʳ�κ������DZ��ϼӵ�ʳ��������һ����Ҫ������ijѧ����ʵ���Ҳⶨʳ�þ������Ƿ⼰�ⶨ����������Ҫ��ӳΪ��
�ӵ�ʳ������ʳ�������ӵ���أ���ʳ�κ���������I���㣩����20��50mg/kg�������������Σ�������Լ��ʳ�κ������DZ��ϼӵ�ʳ��������һ����Ҫ������ijѧ����ʵ���Ҳⶨʳ�þ������Ƿ⼰�ⶨ����������Ҫ��ӳΪ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�021
A�������к͵ζ�ʱ��ֱ�����ϴ�Ӹɾ�����ƿ�мӴ���Һ
B���ڲⶨ�����ᾧˮ����ʱ�����ڸ���������ȴ�������������ƽ���̣��������̷������룬ͬʱ�ƶ�����ʹ��ƽƽ��
C��ijѧ��Ҫ����0��1mol•L��1��ϡH2SO4100mL����������ˮ��������ƿ�̶���2cm��3cmʱ�����ý�ͷ�ιܵμӣ��������ʹҺ�����Գ����̶���һ�㣬�����ಿ��������ʹ��Һ����͵���̶�������
D�����ʵ���Ũ��Ϊ0��05mol��L��1��NaOH����Һ��������Ӵ�����������CO2���ô˱�Һ�ζ�δ֪Ũ�ȵ�HCl��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�021
���в���������ʵ�������ǣ�����
A�������к͵ζ�ʱ��ֱ�����ϴ�Ӹɾ�����ƿ�мӴ���Һ
B���ڲⶨ�����ᾧˮ����ʱ�����ڸ���������ȴ�������������ƽ���̣��������̷������룬ͬʱ�ƶ�����ʹ��ƽƽ��
C��ijѧ��Ҫ����0��1mol•L��1��ϡH2SO4100mL����������ˮ��������ƿ�̶���2cm��3cmʱ�����ý�ͷ�ιܵμӣ��������ʹҺ�����Գ����̶���һ�㣬�����ಿ��������ʹ��Һ����͵���̶�������
D�����ʵ���Ũ��Ϊ0��05mol��L��1��NaOH����Һ��������Ӵ�����������CO2���ô˱�Һ�ζ�δ֪Ũ�ȵ�HCl��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.�к͵ζ�ʱ���ø�ϴ���ĵζ��ܺ���ƿ��ȡ�ô���Һ20.00 mL���еζ�
B.�ζ�δ֪Ũ���������õ�0.1 mol��L-1 NaOH��Һ�������Ʊ�Һʱ��������Ӵ�������������CO2
C.�ñ�NaOH��Һ�ζ�δ֪Ũ�ȵ�ϡH2SO4ʱ���÷�̪��ָʾ��
D.�ζ����ڵζ�ǰ���첿�������ݣ��ڶ��������ݼ�����������ʼ�ζ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com