
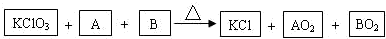
 ���ʴ�Ϊ��
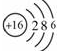
���ʴ�Ϊ��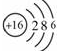 ��
��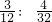 =2��1������ԭ���غ���ƽ��ѧ����ʽ�õ���2KClO3+2C+S=2KCl+2CO2+SO2��
=2��1������ԭ���غ���ƽ��ѧ����ʽ�õ���2KClO3+2C+S=2KCl+2CO2+SO2��

 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
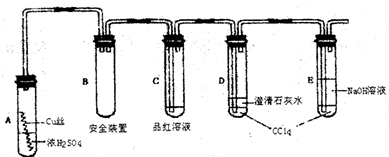
| ||
| ||
| ||
 Cu��OH��2+2H+
Cu��OH��2+2H+ Cu��OH��2+2H+
Cu��OH��2+2H+�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ||
| ||
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�걱���в�ƽ��������ѧ����ĩ�������ۻ�ѧ�Ծ��������棩 ���ͣ������
�������������������й㷺Ӧ�á�
��ҵ�����ؾ�ʯ����Ҫ�ɷ�BaSO4��Ϊԭ���Ʊ�BaCl2���乤������ʾ��ͼ���£�
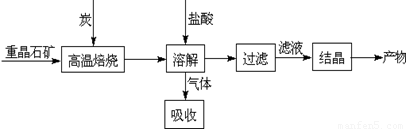
ij�о�С��������ϵã�
BaSO4��s��+4C��s�� 4CO��g��+BaS��s����H1=+571.2kJ•mol-1
��
4CO��g��+BaS��s����H1=+571.2kJ•mol-1
��
BaSO4��s��+2C��s�� 2CO2��g��+BaS��s����H2=+226.2kJ•mol-1
��
2CO2��g��+BaS��s����H2=+226.2kJ•mol-1
��
��1���ù���NaOH��Һ�������壬�õ����ơ��÷�Ӧ�����ӷ���ʽ�� ��
��2����ӦC��s��+CO2��g�� 2CO��g���ġ�H= ��
2CO��g���ġ�H= ��
��3��ʵ�������б�����������̿��ͬʱ��Ҫͨ���������Ŀ��������
�ٴ�ԭ�ϽǶȿ��� ��
�ڴ������Ƕȿ����٢�Ϊ���ȷ�Ӧ��̿��������Ӧ����ά�ַ�Ӧ������¡�
��4����С��ͬѧ���BaSO4��ˮ�еij����ܽ�ƽ������һ���о��������Ϸ�����ij�¶�ʱBaSO4��ˮ�еij����ܽ�ƽ��������ͼ��ʾ��
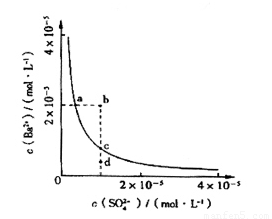
��С��ͬѧ����������ֹ۵㣺
�ٵ�����SO42-����Һ�м���Ba2+ ʹSO42-������ȫ�����ʱSO42-����Һ�е�Ũ��Ϊ0
�ڼ���Na2SO4����ʹ��Һ��a��䵽b��
��ͨ����������ʹ��Һ��d��䵽c��
��d����BaSO4��������
������ȷ���� ������ţ���
��ijȼ�ϵ����CaHSO4����Ϊ����ʴ���H+��������ṹ��ͼ��ʾ������ܷ�Ӧ�ɱ�ʾΪ2H2+O2�T2H2O��
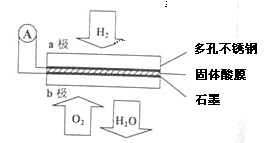
��ش�
��5��H+�� ��ͨ�����������ʴ��ݵ���һ������a����b����
��6��b���Ϸ����ĵ缫��Ӧ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 Cu2S����ɫ��
Cu2S����ɫ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com