
|
�� 1 L���ܱ������У�����1 mol��CO��1 mol��H2O(g)����850��ʱ���л�ѧ��Ӧ��CO(g)��H2O(g)
| |
A�� |
ǰ 4 min����CO��ʾ�Ļ�ѧ��Ӧ����Ϊv(CO)��0.25 mol/(L��min) |
B�� |
��Ӧ�ڵ� 4 minʱ���ڻ�ѧƽ��״̬ |
C�� |
8����ʱ�����������CO2���������Ϊ16�� |
D�� |
t����ʱ����Ӧ�������¶ȸ���850�� |
 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д� ��У������Ԫͬ��ѵ��������ϵ�д�
��У������Ԫͬ��ѵ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
N2��g��+3H2(g) ![]() 2NH3(g);��H��0
2NH3(g);��H��0

�����ͼʾ�ش��������⣺
��1����Ӧ��ʼʱ�����������������ʵ���֮��n(N2)��n(H2)= _________��_________����15����ʱ�ϳɰ���Ӧ��һ�δﵽƽ�⣬�����H2��Ũ�ȱ仯����ʾ��ƽ����ѧ��Ӧ����Ϊ_________________________��
��2����t1ʱ�����߷����仯��ԭ����__________________��__________________�������������t1���ʱ�����꣨_________��_________���Լ���t2ƽ��ʱ�����꣨_________��_________��������ͼ�л�����t1��t2֮�����������ʵ����仯���ߡ�
��3��Ϊ�ﵽͼʾ��t2��ƽ��״̬����t1��t2֮����Ҫ��ȡ�Ĵ�ʩ��_________��
a.�������������
b.�����¶�
c.�����¶�
d.�����������
��4������ʮ��������ѭ�����̣�������t11�ﵽƽ��ʱ��N2��H2�����ʵ���֮��n(N2)��n(H2)= _________��_________,����������N2��H2����ת����֮�Ȧ�(N2)�æ�(H2)= _________��_________��
��5���������ϼ����������㽨��ϳɰ���Ӧ�����ԭ�ϱ��ǣ�n(N2)��n(H2)=_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�긣��ʡ���������ѧУ����ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ������
��15�֣�A��B��C��D��E��F��ԭ�Ӱ뾶���μ�С�Ķ�����Ԫ�أ�A��C��Ԫ���γɵĻ������dz�������õĵ�ζƷ��A��B��C������������Ӧ��ˮ������������ܷ�Ӧ�����κ�ˮ��DԪ�ص�ͬλ��֮һ��������Ϊ���ԭ�������Ͱ����ӵ������ı���EԪ�����γɶ���ͬ���� ���壬����һ���ǵ�������ʳƷ��ҩƷ���ʵ����壻FԪ�ص��������������л���ȼ�յ�Һ̬������ݴ˻ش�
���壬����һ���ǵ�������ʳƷ��ҩƷ���ʵ����壻FԪ�ص��������������л���ȼ�յ�Һ̬������ݴ˻ش�
��1��A��C��E��ԭ�Ӹ���֮��1 :1 :1�γɵĻ�������һ�ֳ�������������������������ˮ��Һ����pH��ֽ�ϣ��ɹ۲쵽������ ��ԭ���� ��
��2���ҹ��״��ĺ�������B�ĵ���Ϊ����������Ϊ����������������������ˮ���������Һ����ص�������ӦʽΪ  ������ܷ�Ӧ�Ļ�ѧ����ʽΪ ��
������ܷ�Ӧ�Ļ�ѧ����ʽΪ ��
��3��C�����������Ϊ��ɫҺ�壬0.25 mol ��������һ����ˮ��ϵõ�һ��ϡ��Һ�����ų�Q kJ ��������д���÷�Ӧ���Ȼ�ѧ����ʽ�� ____________________________________��
��4����1 L���ܱ������У�����1 mo1DE��1 mo1 F2E (g)����850��ʱ���л�ѧ��Ӧ��DE (g) + F2E (g) DE2(g) + F2(g) ��H��0����ƽ��ʱ����50%
DE2(g) + F2(g) ��H��0����ƽ��ʱ����50% ��DEת��ΪDE2������ͬ�¶��£���1 mo1DE��4 mo1 F2E (g)����ͬ���������У���¼0~8�����ڲ�������ڸ����ʵ����ʵ������±���t����ʱΪ�ı��������ƽ��ʱ����õ����ݡ�����˵����ȷ����
��DEת��ΪDE2������ͬ�¶��£���1 mo1DE��4 mo1 F2E (g)����ͬ���������У���¼0~8�����ڲ�������ڸ����ʵ����ʵ������±���t����ʱΪ�ı��������ƽ��ʱ����õ����ݡ�����˵����ȷ����
| ʱ��/min | n(DE)/ mo1 | n(F2E)/mo1 | n(DE2)/ mo1 | n(F2)/ mo1 |
| 0 | 1 | 4 | 0 | 0 |
| 4 | 0.25 | 3.25[��Դ:ѧ���ơ���Z��X��X��K] | 0.75 | 0.75 |
| 6 | n1 | n2 | n3 | n4 |
| 8 | n1 | n2 | n3 | n4 |
| t | 0.15 | 3.15 | 0.85 | 0.85 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ������ѧ����У������һ��������ѧ�Ծ����������� ���ͣ������
����10�֣��״��ϳɷ�Ӧ���������仯��ͼ��ʾ��
��1��д���ϳɼ״����Ȼ�ѧ����ʽ________________________________��
ʵ������1 L���ܱ������н���ģ��ϳ�ʵ�顣��1 mol CO��2 mol H2ͨ�������У��ֱ������300 ���500 �淴Ӧ��ÿ��һ��ʱ����������CH3OH��Ũ�����±���ʾ��
| ʱ��Ũ��(mol/L)�¶� | 10 min | 20 min | 30 min | 40 min | 50 min | 60 min |
| 300 �� | 0.40 | 0.60 | 0.75 | 0.84 | 0.90 | 0.90 |
| 500 �� | 0.60 | 0.75 | 0.78 | 0.80 | 0.80 | 0.80 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�걱���г�����������һ���ۺ���ϰ�����ۣ���ѧ���� ���ͣ�ѡ����
��֪��
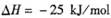 ��ij�¶��µ�ƽ�ⳣ��Ϊ400�����¶��£���1 L���ܱ������м���CH3OH,��Ӧ��ijʱ�̲�ø���ֵ����ʵ���Ũ�����£�
��ij�¶��µ�ƽ�ⳣ��Ϊ400�����¶��£���1 L���ܱ������м���CH3OH,��Ӧ��ijʱ�̲�ø���ֵ����ʵ���Ũ�����£�

����˵����ȷ����
A. ƽ��������¶ȣ�ƽ�ⳣ��>400
B. ƽ��ʱ��c(CH3OCH3)=1.6 mol/L
C. ƽ�ⅼ����Ӧ����������������20kJ
D ƽ��ʱ���ټ�������ʼ������CH3OH,����ƽ���CH3OHת��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�긣��ʡ����ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ������
��15�֣�A��B��C��D��E��F��ԭ�Ӱ뾶���μ�С�Ķ�����Ԫ�أ�A��C��Ԫ���γɵĻ������dz�������õĵ�ζƷ��A��B��C������������Ӧ��ˮ������������ܷ�Ӧ�����κ�ˮ��DԪ�ص�ͬλ��֮һ��������Ϊ���ԭ�������Ͱ����ӵ������ı���EԪ�����γɶ���ͬ�������壬����һ���ǵ�������ʳƷ��ҩƷ���ʵ����壻FԪ�ص��������������л���ȼ�յ�Һ̬������ݴ˻ش�
��1��A��C��E��ԭ�Ӹ���֮��1 :1 :1�γɵĻ�������һ�ֳ�������������������������ˮ��Һ����pH��ֽ�ϣ��ɹ۲쵽������ ��ԭ���� ��
��2���ҹ��״��ĺ�������B�ĵ���Ϊ����������Ϊ����������������������ˮ���������Һ����ص�������ӦʽΪ ������ܷ�Ӧ�Ļ�ѧ����ʽΪ ��
��3��C�����������Ϊ��ɫҺ�壬0.25 mol ��������һ����ˮ��ϵõ�һ��ϡ��Һ�����ų�Q kJ ��������д���÷�Ӧ���Ȼ�ѧ����ʽ�� ____________________________________��
��4����1 L���ܱ������У�����1 mo1DE��1 mo1 F2E (g)����850��ʱ���л�ѧ��Ӧ��DE (g) + F2E (g) DE2(g)
+ F2(g) ��H��0����ƽ��ʱ����50%��DEת��ΪDE2������ͬ�¶��£���1 mo1DE��4
mo1 F2E (g)����ͬ���������У���¼0~8�����ڲ�������ڸ����ʵ����ʵ������±���t����ʱΪ�ı��������ƽ��ʱ����õ����ݡ�����˵����ȷ����
DE2(g)
+ F2(g) ��H��0����ƽ��ʱ����50%��DEת��ΪDE2������ͬ�¶��£���1 mo1DE��4
mo1 F2E (g)����ͬ���������У���¼0~8�����ڲ�������ڸ����ʵ����ʵ������±���t����ʱΪ�ı��������ƽ��ʱ����õ����ݡ�����˵����ȷ����
|
ʱ��/min |
n(DE)/ mo1 |
n(F2E)/mo1 |
n(DE2)/ mo1 |
n(F2)/ mo1 |
|
0 |
1 |
4 |
0 |
0 |
|
4 |
0.25 |
3.25[��Դ:ѧ���ơ���Z��X��X��K] |
0.75 |
0.75 |
|
6 |
n1 |
n2 |
n3 |
n4 |
|
8 |
n1 |
n2 |
n3 |
n4 |
|
t |
0.15 |
3.15 |
0.85 |
0.85 |
�ٷ�Ӧ�ڵ�4 minʱ��v������ v���棩�����������������������
��8����ʱ�����������DE2���������Ϊ ��
��t����ʱ����Ӧ�������¶� 850�棨���������������������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com