
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����KMnO4��Һ���/mL | 26.42 | 25.05 | 24.95 |
 2Fe2O3+8SO2��
2Fe2O3+8SO2�� 2Fe2O3+8SO2��
2Fe2O3+8SO2��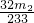 g
g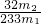 ��100%��
��100%��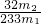 ��100%�������������𰸣�
��100%�������������𰸣�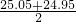 mL=25.00ml��
mL=25.00ml��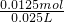 =0.5mol/L��
=0.5mol/L��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||


| 32m2 |
| 233m1 |
| 32m2 |
| 233m1 |

| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����KMnO4��Һ���/mL | 26.42 | 25.05 | 24.95 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


| ||
| ||
| 32m2 |
| 233m1 |
| 32m2 |
| 233m1 |
| 28V |
| m1 |
| 28V |
| m1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ�人�и������µ��в��������ۺϻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
���������ҹ���������᳧��ȡ�������Ҫԭ�ϡ�ij��ѧ��ȤС���ij������ʯ����Ҫ �ɷ�ΪFeS2)������Ԫ�غ����ⶨ��ʵ��̽������ҵ���������̽����
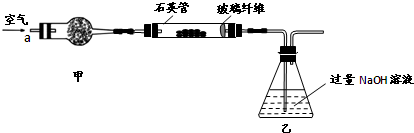
I ����m1,g�û�������Ʒ�������в������������������ͼ��ʾװ�ã��гֺͼ���װ�� ʡ�ԣ���ʯӢ����,��a�����ϵػ���ͨ���������������ʯӢ���еĻ�������Ʒ����Ӧ��ȫ��ʯӢ���з�����Ӧ�Ļ�ѧ����ʽΪ��4FeS2 + 11O2  2Fe2O3
+ 8SO2
2Fe2O3
+ 8SO2

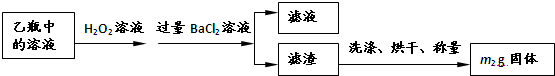
II��Ӧ������,����ƿ�е���Һ�������´���

��1��I�У���ƿ�ڷ�����Ӧ�����ӷ���ʽ��____________��__________��
��2��II�У�����H2O2��Һ��������������____________________________
��3���û�����ʯ����Ԫ�ص���������Ϊ____________________________
��4�������ڴ���Ӧ���������Ƚ�������Ŀ�ģ�______________��
��5����ҵ�����г��ð��������ᷨ����β�������Դﵽ������Ⱦ���������õ�Ŀ��,�� ������ѧ����ʽ��ʾ�䷴Ӧԭ��_______________��______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������ʡ��У������һ��ģ�⿼�����ۻ�ѧ���� ���ͣ��ۺ���
��14�֣����������ҹ���������᳧��ȡ�������Ҫԭ�ϡ�ij��ѧ��ȤС���ij������ʯ����Ҫ�ɷ�ΪFeS����������ʵ��̽����
[ʵ��һ]���ⶨ��Ԫ�صĺ���
I����mg�û�������Ʒ�������в������������������ͼ��ʾװ�ã��гֺͼ���װ��ʡ�ԣ���ʯӢ���У���a�����ϵػ���ͨ�˿�������������ʯӢ���еĻ�������Ʒ����Ӧ��ȫ��ʯӢ���з�����Ӧ�Ļ�ѧ����ʽΪ��

��Ӧ��������ƿ�е���Һ�������´�����

�������ۣ�
��1��I�У���ƿ����ʢ�Լ��ǣߣߣߣߣߣߣߣ���Һ����ƿ�ڷ�����Ӧ�����ӷ���ʽ�Уߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ��ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��2�����С�����HO��Һ�������������ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��3���û�����ʯ����Ԫ�ص���������Ϊ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
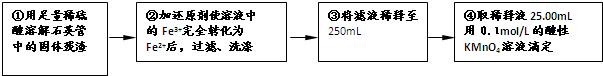
[ʵ���]���������ʵ�鷽���ⶨ��Ԫ�صĺ���

�������ۣ�
��4�����У���ѡ����������ԭ��������Ϊ������? �ߣߣߣߣߣߣߣߡ����������������Ӱ�����������ߣߣߣߣߣߣߣߣ����������˿ղ��𣩡�
��5�����У���Ҫ�õ����������ձ�������������ͷ�ι��⣬���Уߣߣߣߣߣߣߣߡ�
��6�����и��������ҺӦ���ڣߣߣߣߣߵζ����У��жϵζ��յ������Ϊ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com