
���� ��1������Ũ��Һ��ϡ��ǰ�����ʵ����ʵ���������㣻
��2���ٸ��ݰѲ���������������������ƿ�̶������ϻ����ʲôӰ���Լ������������÷�����
�ڴ�ϴ��Һ����������ƿ�ܷ���������з�����
�۸���Һ�����״������
�ܸ���ABCѡ���ܷ���������з�����
��3��������Ͳ�����á���ˮ�����������к�Σ�շ�����
��� �⣺��1������C1V1=C2V2�ã�V1=$\frac{{C}_{2}{V}_{2}}{{C}_{1}}$=$\frac{0.1��3.6}{18}$=20.0ml���ʴ�Ϊ20.0mL��
��2����������������������ƿ�̶������ϣ���ʹ������Һ�����ڿ̶������϶����¶���ʱ��ƫ���������ƿ����ϸ��Ϊ������Һ��������Ӧ�ò������������ʴ�Ϊ������������������ƿ�̶������£�ʹ��Һ�ز����������ص�������ƿ�У�
��ϴ��Һ�к��в������ʣ�����ϴ��Һ��������ƿ�У��ᵼ����ҺŨ�Ƚ��ͣ��ʴ�Ϊ��ʹ������ȫת�Ƶ�����ƿ�У�
�ۼ�ˮ����̶���1��2cmʱ���ý�ͷ�ιܵμ�ˮ��������ˮ�������Һ���ǰ��εģ�ֻ��Һ����Ͷ���̶�������ʱ��������Һ����ʵ������������Ũ����ƫ��ʴ�Ϊ����ˮ����̶���1��2cmʱ�����ý�ͷ�ιܵμ�ˮ��Һ����̶������У�
���ý�ͷ�ι�������ƿ�м�ˮʱ����С��Һ�泬���˿̶ȣ����۲�ȡA��B��C�ĺ��ַ�ʽ���������ó���Ҫ����Һ��ֻ���������ã��ʴ�Ϊ��D��
��3����Ͳֻ����ȡҺ�岻��������Һ�������ˮ����Ũ�����л����Һ��ɽ����ʴ�Ϊ������ȷ����������Ͳ������Һ�����ܽ�ˮ���뵽Ũ�����У�
���� ���Ũ��ƫ��ƫС��������������C=n/V�����ж������ʸı仹����Һ�ı䣬�Ӷ��ж�Ũ��ƫ����ƫС��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
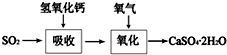 ��ҵ�ϳ����������ķ�������ˮ�����������ȣ�����������ã������ŷţ��������Ⱦ���������ѧ���գ��ɱ��Ϊ����
��ҵ�ϳ����������ķ�������ˮ�����������ȣ�����������ã������ŷţ��������Ⱦ���������ѧ���գ��ɱ��Ϊ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
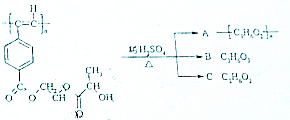 ����Ȳ����ۺ���ĺϳ�ʹ�߷��Ӳ��Ͻ����ˡ��ϳɽ����������ϵ���ѧʱ������ͼ�Ǿ���Ȳ���������M�Ľṹʽ��M��ϡ���������µ�ˮ��ʾ��ͼ��
����Ȳ����ۺ���ĺϳ�ʹ�߷��Ӳ��Ͻ����ˡ��ϳɽ����������ϵ���ѧʱ������ͼ�Ǿ���Ȳ���������M�Ľṹʽ��M��ϡ���������µ�ˮ��ʾ��ͼ��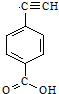 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʹ������ƿǰ�������Ƿ�©ˮ | |
| B�� | ������ˮע������ƿ�У�Һ����̶�����1-2cmʱ�����ý�ͷ�ιܵμ���Һ����̶������� | |
| C�� | ������Һʱ������Ͳ��ȡ������ֱ�ӵ�������ƿ�У�������������ˮ���̶��� | |
| D�� | ���ݺ�Ǻ�ƿ�����������µߵ���ҡ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ʪ���pH��ֽ��ϡ��Һ��pHֵ���ⶨֵƫС | |
| B�� | ������ƿ������Һ������ʱ���ӿ̶��ߣ�������ҺŨ��ƫ�� | |
| C�� | ���������Һ�ζ�δ֪Ũ�ȵ�NaOH��Һʱ�����ζ�ǰ�ζ����������ݣ��յ����ʱ�����ݣ�������NaOH��Һ��Ũ��ƫ�� | |
| D�� | �ⶨ�кͷ�Ӧ�ķ�Ӧ��ʱ��������������У������¶�ֵƫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.30 | B�� | 0.35 | C�� | 0.40 | D�� | 0.50 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com