
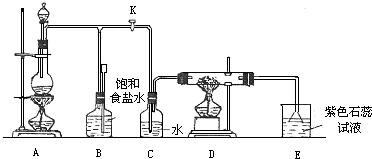
(��)ʵ�����������������ʵķ�����������ȷ����________(�����)
������ᱣ����ϸ�ڲ���ƿ��
��Ũ��������ɫƿʢ�ţ�������������
������Һ�����ˮ��棬��ֹ��ӷ�
������������Һ�����ڴ�ĥ�ڲ������IJ����Լ�ƿ��
����ʵ���������Ľ����Ʊ�����ú����
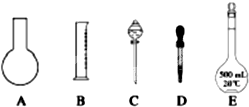
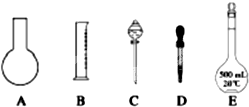
(��)ʵ������ȡ0.5 mol/L��NaCl��Һ500 mL��������������
���ձ�
��100 mL��Ͳ
��1000 mL����ƿ
��500 mL����ƿ
�ݲ�����
��������ƽ(������)
(1)����ʱ������ʹ�õ�������________(�����)����ȱ�ٵ�������________��
(2)ʵ�������õ��������������÷ֱ���________��________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ѧ��һ����ʵ��Ϊ������ѧ�ƣ�����ʹ����ѧ��ѧʵ���еij�����������Ϥ����ʵ����Ʒ����ȷ����ʵ��Ļ������Իش��������⣺
��ѧ��һ����ʵ��Ϊ������ѧ�ƣ�����ʹ����ѧ��ѧʵ���еij�����������Ϥ����ʵ����Ʒ����ȷ����ʵ��Ļ������Իش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ѧ��һ����ʵ��Ϊ������ѧ�ƣ�����ʹ����ѧ��ѧʵ���еij�����������Ϥ����ʵ����Ʒ����ȷ����ʵ��Ļ������Իش��������⣺
��ѧ��һ����ʵ��Ϊ������ѧ�ƣ�����ʹ����ѧ��ѧʵ���еij�����������Ϥ����ʵ����Ʒ����ȷ����ʵ��Ļ������Իش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ���������۶��С���ɽ��ѧ��������ѧ�����߶����ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com