
 �����ʡ�
�����ʡ�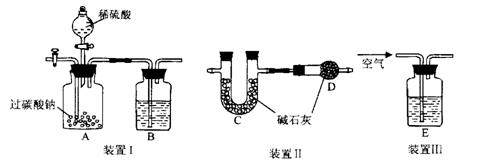
 ֤�������������壬B��ʢ��������Ba��OH��2��Һ�����۲쵽�������� ����֤���� �������ɣ�������֤��һ������ķ��� ��
֤�������������壬B��ʢ��������Ba��OH��2��Һ�����۲쵽�������� ����֤���� �������ɣ�������֤��һ������ķ��� �� װ��III��ͨ������Ŀ���� ��
װ��III��ͨ������Ŀ���� ��
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
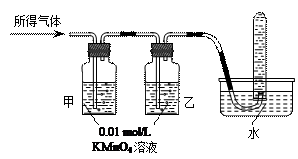 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��С�����ʵ��̽�����Ļ���������ʣ�װ������ͼ��ʾ(Aװ��δ����)������AΪ���巢��װ�á�A�������Լ��������й���������ѡȡ����NH4HCO3����NH4Cl����Ca(OH)2����NaOH��
��С�����ʵ��̽�����Ļ���������ʣ�װ������ͼ��ʾ(Aװ��δ����)������AΪ���巢��װ�á�A�������Լ��������й���������ѡȡ����NH4HCO3����NH4Cl����Ca(OH)2����NaOH��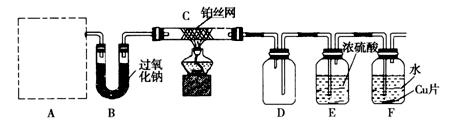
 __��
__�� ____________________________________________________________��
____________________________________________________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ���� | ʵ����� | Ԥ�������� |
| 1 | ȡ������ɫ��Һ������NaOH��Һ | �����ɺ��ɫ�����������ȷ |
| 2 | ȡ������ɫ��Һ���������KI��Һ | ����Һ����ɫ��������ȷ |
| 3 | ȡ������ɫ��Һ�����뱽��Һ������ | ���ϲ���Һ�ʳȺ�ɫ���� ��ȷ |
 �������ӷ���ʽ��ʾ����
�������ӷ���ʽ��ʾ����| ʵ����� | Ԥ�������� |
| | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
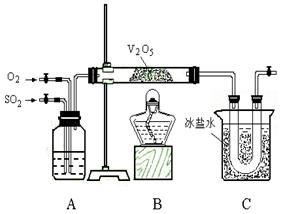
 ��ʱ�õ�b g ��������ʵ���ж��������ת���ʲ�С��_________________��
��ʱ�õ�b g ��������ʵ���ж��������ת���ʲ�С��_________________�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
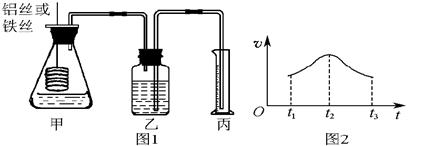
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
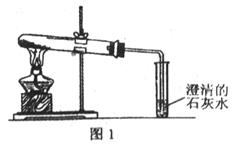
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
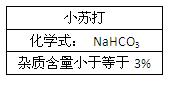
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
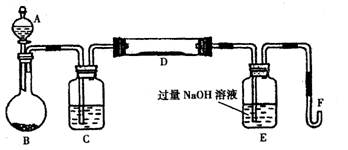
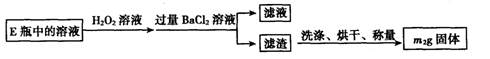
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com