
��15�֣���Ԫ�ص��⻯����������ڹ�ҵ�������������ж��й㷺��Ӧ�á���ش��������⣺
��1����������N2��H2��Ӧ��ȡ��N2��g��+3H2 ��g�� 2NH3��g����Ӧ���̵������仯��ͼ��ʾ��
2NH3��g����Ӧ���̵������仯��ͼ��ʾ��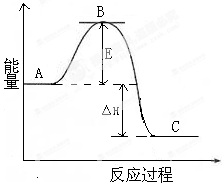
��֪��1molN2�����еĻ�ѧ������ʱ��Ҫ����942kJ��������1molH2�����еĻ�ѧ������ʱ��Ҫ����436kJ���������γ�1mol N��H��ʱ�ͷ�390.8kJ��������
��ͼ��A��C�ֱ��ʾ �� ��E�Ĵ�С�Ը÷�Ӧ �ķ�Ӧ������Ӱ�죿 ��
�÷�Ӧ��Ҫ������ý��������������ý��ʹͼ��B�������ǽ��ͣ� ���� ��
��ͼ�С�H= kJ��mol-1��
�������Ӧ���ʦͣ�H2��Ϊ0.15mol�� L-1��min-1,��ͣ�N2��= mol�� L-1��min-1���ͣ�NH3��=
mol��L-1 ��min-1��
��2���¿���Ϊ�����������ȼ�ϣ���������N2O4��Ӧ����N2��ˮ������
��֪��N2��g��+2O2��g��=N2O4��l�� ��H= -19.5kJ��mol-1
N2H4��l��+O2��g��= N2��g��+2H2O��g����H= -534.2kJ��mol-1
��д���º� N2O4��Ӧ���Ȼ�ѧ��Ӧ����ʽ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������ѧ2012������ڶ����¿���ѧ���� ���ͣ�058
A��
��������(NF3)��һ����ɫ����ζ�����Ҳ���ȼ�����壬�ڰ뵼��ӹ���̫���ܵ�������Һ����ʾ�������еõ��㷺Ӧ�ã�������ͭ�Ĵ���������F2������NH3��Ӧ�õ����÷�Ӧ��һ�ֲ���Ϊ�Σ�(1)�÷�Ӧ�Ļ�ѧ����ʽΪ________��������NF3�е�ԭ�ӵ��ӻ���ʽΪ________��NF3���ӿռ乹��Ϊ________��
(2)N��F����Ԫ�ص��⻯���ȶ��ԱȽϣ�NH3________HF(ѡ���������)��
(3)N3������Ϊ��±���ӣ�д��1����N3����Ϊ�ȵ�����ķ��ӵĻ�ѧʽ________��
(4)Ԫ��A��̬ԭ�ӵĺ�������Ų�ʽΪ
1s22s22p63s23p64s2��A�������γ����ӻ�����侧���ṹ��ͼ�������ӻ�����Ļ�ѧʽΪ________��
B���̷�(FeSO4��7H2O)���������������ʵ�����Ͽ��Ƶ�Ħ���ξ��壬��Ӧԭ��Ϊ��
(NH4)2SO4��FeSO4��6H2O��(NH4)2SO4��FeSO4��6H2O���������̿ɱ�ʾΪ��
(1)ϴ����Na2CO3����Ҫ������________��
(2)�ᾧ������Ҫ���������ܼ���Ũ���ᾧ��Ӧ���ȵ�________ʱ��ֹͣ���ȣ�
(3)����������ͼ��ʾװ�ý��еģ����ֹ��˸���ͨ������ȣ����˹����ٶȿ��⣬����һ���ŵ���________��
(4)����ˮ�Ҵ�ϴ�ӵ�Ŀ����________��
(5)��Ʒ��Fe2+�Ķ����������Ƶõ�Ħ������Ʒ���������м�������Fe3+��Ϊ�˲ⶨĦ���β�Ʒ��Fe2+�ĺ�����һ�����������������KMnO4��Һ�ζ��ķ�������ȡ4.0 g��Ħ������Ʒ������ˮ������������ϡ���ᣮ��0.2 mol/L��KMnO4��Һ�ζ�������Һ��Fe2+ȫ��������ʱ������KMnO4��Һ10.00 mL��
�ٱ�ʵ���ָʾ����________��(����ĸ)
A����̪
B������
C��ʯ��
D������Ҫ
�ڲ�Ʒ��Fe2+����������Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ������ʡ�����и����������ʼ죨���ۣ���ѧ���� ���ͣ������
��Ԫ�����ڱ��д�������λ�õ�Ԫ���ڽṹ�����������������Ƶĵط����ڶ����ڵ�̼�������������������γ��⻯���Ԫ�ص��⻯���H O�⣬����H
O�⣬����H O
O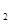 ��̼Ԫ�ص��⻯���CH
��̼Ԫ�ص��⻯���CH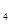 �⣬����C
�⣬����C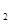 H
H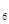 �ȣ���֮���Ƶĵ�Ԫ�ص��⻯����⣬����N
�ȣ���֮���Ƶĵ�Ԫ�ص��⻯����⣬����N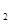 H
H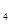 �ȡ�
�ȡ�
��1��̼ԭ��֮����Խ�ϳ���״�ṹ����ԭ��֮��Ҳ�����γ���״�ṹ�����赪ԭ�Ӽ�ֻ�Ե���������ʽ���ӳ���״�����γ��⻯����ϵ���⻯���ͨʽΪ ��
��2����ϵ���е�N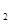 H
H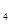 �ǡ�����������ʱ������õ�Һ��ȼ�ϣ�Һ̬������������������������Һ̬ȼ�ϵ��ŵ��Dz�����������������Ⱦ����֪40g N
�ǡ�����������ʱ������õ�Һ��ȼ�ϣ�Һ̬������������������������Һ̬ȼ�ϵ��ŵ��Dz�����������������Ⱦ����֪40g N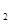 H
H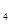 �ڻ������ʱ��Ӧ�зų�710kJ������д���������ʱ�÷�Ӧ���Ȼ�ѧ����ʽ��
��
�ڻ������ʱ��Ӧ�зų�710kJ������д���������ʱ�÷�Ӧ���Ȼ�ѧ����ʽ��
��
��3����ϵ�����е�NH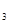 ��ũҵ����ѧ��������ҵ������Ҫ���塣��ϳ�ԭ��Ϊ��
��ũҵ����ѧ��������ҵ������Ҫ���塣��ϳ�ԭ��Ϊ��
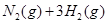

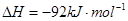
I����һ���¶��£���1.5molN ��6 molH
��6 molH ͨ�뵽һ���̶��ݻ�ΪVL���ܱ������У�����Ӧ�ﵽƽ��ʱ�������������ѹǿΪ��ʼʱ��80%����
ͨ�뵽һ���̶��ݻ�ΪVL���ܱ������У�����Ӧ�ﵽƽ��ʱ�������������ѹǿΪ��ʼʱ��80%����
��ʱ��Ӧ�ų�������Ϊ kJ.
H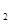 ��ת����= ��
��ת����= ��
���¶��ºϳɰ���Ӧ��ƽ�ⳣ��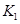 =
��ֻ�����ֱ���ʽ��
=
��ֻ�����ֱ���ʽ��
II���ڱ����¶Ȳ��䣬��ͬ������ܱ������У�����ʼ�����ʵ�����ΪamolN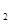 ��bmolH
��bmolH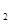 ��cmolNH
��cmolNH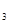 ,ƽ��ʱNH
,ƽ��ʱNH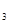 �����ʵ�������Ϊ25%����
�����ʵ�������Ϊ25%����
�ﵽƽ��ʱ��I��II�ų������� ������ĸ���ţ�
A��һ�����
B��ǰ��һ��С�ں���
C��ǰ�ߵ��ڻ�С�ں���
D��ǰ�ߵ��ڻ���ں���
I��II�ϳɰ���ƽ�ⳣ���ֱ�Ϊ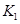 ��
��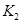 ��ͬ
��ͬ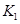
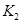 �����������������
��=����
�����������������
��=����
��ʹ��ʼʱ��Ӧ������У�a��ȡֵ��ΧΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��Ԫ�����ڱ��д�������λ�õ�Ԫ���ڽṹ�����������������Ƶĵط����ڶ����ڵ�̼�������������������γ��⻯���Ԫ�ص��⻯���H![]() O�⣬����H
O�⣬����H![]() O
O![]() ��̼Ԫ�ص��⻯���CH
��̼Ԫ�ص��⻯���CH![]() �⣬����C
�⣬����C![]() H
H![]() �ȣ���֮���Ƶĵ�Ԫ�ص��⻯����⣬����N
�ȣ���֮���Ƶĵ�Ԫ�ص��⻯����⣬����N![]() H
H![]() �ȡ�
�ȡ�
��1��̼ԭ��֮����Խ�ϳ���״�ṹ����ԭ��֮��Ҳ�����γ���״�ṹ�����赪ԭ�Ӽ�ֻ�Ե���������ʽ���ӳ���״�����γ��⻯����ϵ���⻯���ͨʽΪ ��
��2����ϵ���е�N![]() H
H![]() �ǡ�����������ʱ������õ�Һ��ȼ�ϣ�Һ̬������������������������Һ̬ȼ�ϵ��ŵ��Dz�����������������Ⱦ����֪40g N
�ǡ�����������ʱ������õ�Һ��ȼ�ϣ�Һ̬������������������������Һ̬ȼ�ϵ��ŵ��Dz�����������������Ⱦ����֪40g N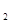 H
H![]() �ڻ������ʱ��Ӧ�зų�710kJ������д���������ʱ�÷�Ӧ���Ȼ�ѧ����ʽ�� ��
�ڻ������ʱ��Ӧ�зų�710kJ������д���������ʱ�÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��3����ϵ�����е�NH![]() ��ũҵ����ѧ��������ҵ������Ҫ���塣��ϳ�ԭ��Ϊ��
��ũҵ����ѧ��������ҵ������Ҫ���塣��ϳ�ԭ��Ϊ��
![]()
![]()
![]()
I����һ���¶��£���1.5molN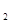 ��6 molH
��6 molH![]() ͨ�뵽һ���̶��ݻ�ΪVL���ܱ������У�����Ӧ�ﵽƽ��ʱ�������������ѹǿΪ��ʼʱ��80%����
ͨ�뵽һ���̶��ݻ�ΪVL���ܱ������У�����Ӧ�ﵽƽ��ʱ�������������ѹǿΪ��ʼʱ��80%����
��ʱ��Ӧ�ų�������Ϊ kJ.
H![]() ��ת����= ��
��ת����= ��
���¶��ºϳɰ���Ӧ��ƽ�ⳣ��![]() = ��ֻ�����ֱ���ʽ��
= ��ֻ�����ֱ���ʽ��
II���ڱ����¶Ȳ��䣬��ͬ������ܱ������У�����ʼ�����ʵ�����ΪamolN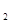 ��bmolH
��bmolH![]() ��cmolNH
��cmolNH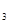 ,ƽ��ʱNH
,ƽ��ʱNH![]() �����ʵ�������Ϊ25%����
�����ʵ�������Ϊ25%����
�ﵽƽ��ʱ��I��II�ų������� ������ĸ���ţ�
A��һ�����
B��ǰ��һ��С�ں���
C��ǰ�ߵ��ڻ�С�ں���
D��ǰ�ߵ��ڻ���ں���
I��II�ϳɰ���ƽ�ⳣ���ֱ�Ϊ![]() ��
��![]() ��ͬ
��ͬ![]()
![]() �����������������=����
�����������������=����
��ʹ��ʼʱ��Ӧ������У�a��ȡֵ��ΧΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011������ʡ�����и����������ʼ죨���ۣ���ѧ���� ���ͣ������
��Ԫ�����ڱ��д�������λ�õ�Ԫ���ڽṹ�����������������Ƶĵط����ڶ����ڵ�̼�������������������γ��⻯���Ԫ�ص��⻯���H O�⣬����H
O�⣬����H O
O ��̼Ԫ�ص��⻯���CH
��̼Ԫ�ص��⻯���CH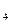 �⣬����C
�⣬����C H
H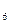 �ȣ���֮���Ƶĵ�Ԫ�ص��⻯����⣬����N
�ȣ���֮���Ƶĵ�Ԫ�ص��⻯����⣬����N H
H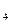 �ȡ�
�ȡ�
��1��̼ԭ��֮����Խ�ϳ���״�ṹ����ԭ��֮��Ҳ�����γ���״�ṹ�����赪ԭ�Ӽ�ֻ�Ե���������ʽ���ӳ���״�����γ��⻯����ϵ���⻯���ͨʽΪ ��
��2����ϵ���е�N H
H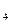 �ǡ�����������ʱ������õ�Һ��ȼ�ϣ�Һ̬������������������������Һ̬ȼ�ϵ��ŵ��Dz�����������������Ⱦ����֪40g N
�ǡ�����������ʱ������õ�Һ��ȼ�ϣ�Һ̬������������������������Һ̬ȼ�ϵ��ŵ��Dz�����������������Ⱦ����֪40g N H
H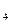 �ڻ������ʱ��Ӧ�зų�710kJ������д���������ʱ�÷�Ӧ���Ȼ�ѧ����ʽ�� ��
�ڻ������ʱ��Ӧ�зų�710kJ������д���������ʱ�÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��3����ϵ�����е�NH ��ũҵ����ѧ��������ҵ������Ҫ���塣��ϳ�ԭ��Ϊ��
��ũҵ����ѧ��������ҵ������Ҫ���塣��ϳ�ԭ��Ϊ��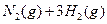

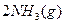
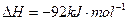
I����һ���¶��£���1.5molN ��6 molH
��6 molH ͨ�뵽һ���̶��ݻ�ΪVL���ܱ������У�����Ӧ�ﵽƽ��ʱ�������������ѹǿΪ��ʼʱ��80%����
ͨ�뵽һ���̶��ݻ�ΪVL���ܱ������У�����Ӧ�ﵽƽ��ʱ�������������ѹǿΪ��ʼʱ��80%����
��ʱ��Ӧ�ų�������Ϊ kJ.
H ��ת����= ��
��ת����= ��
���¶��ºϳɰ���Ӧ��ƽ�ⳣ��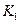 = ��ֻ�����ֱ���ʽ��
= ��ֻ�����ֱ���ʽ��
II���ڱ����¶Ȳ��䣬��ͬ������ܱ������У�����ʼ�����ʵ�����ΪamolN ��bmolH
��bmolH ��cmolNH
��cmolNH ,ƽ��ʱNH
,ƽ��ʱNH �����ʵ�������Ϊ25%����
�����ʵ�������Ϊ25%����
�ﵽƽ��ʱ��I��II�ų������� ������ĸ���ţ�
| A��һ����� |
| B��ǰ��һ��С�ں��� |
| C��ǰ�ߵ��ڻ�С�ں��� |
| D��ǰ�ߵ��ڻ���ں��� |
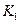 ��
��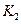 ��ͬ
��ͬ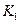
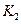 �������������
������������� ����
���� ��=����
��=�����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com