
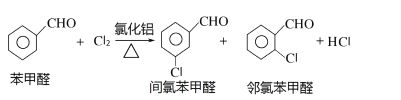
����Ŀ�����ȱ���ȩ������ũҩ��ҽҩ���л��ϳɵ��м��壬����Ҫ���л�������Ʒ��ʵ������ȡ���ȱ���ȩ�Ĺ������¡�
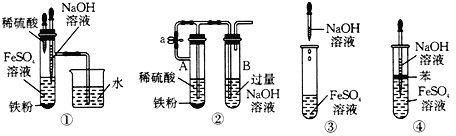
��Cl2���Ʊ�
���շ��������ķ�����������ʵ�����Ʊ���������ʵ�������ø÷����Ʊ�Cl2��
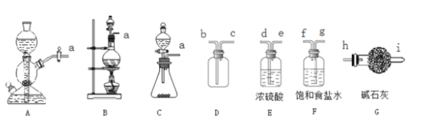
��1���÷�������ѡ��ͼ�е�_____������ĸ��ţ�ΪCl2����װ�ã���Ӧ���������ͻ�ԭ�������ʵ���֮��Ϊ_______��
��2��ѡ��ͼ�е�װ���ռ�һƿ���������Cl2���ӿڵ�����˳��Ϊa��___________��������������Сд��ĸ��ţ�
��3������ƽ���ƶ�ԭ�������ñ���ʳ��ˮ��ȥCl2�л��е�HCl��ԭ��_____________________��
���ȱ���ȩ���Ʊ�
��Ӧԭ����
ʵ��װ�ú�������ͼ��ʾ��
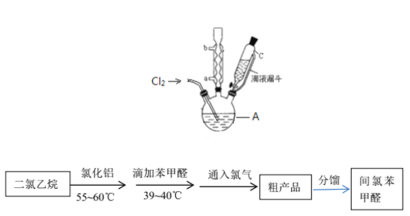
��4��ͼ������A��������_____________��
��5�����ȱ���ȩ���Ʊ������У����������������________________________����ʵ��Ҫ����ˮ������������____________________________________________����ʵ�������κ��¹��̣�Ϊ���Ʒ�Ӧ�¶�����A���ɲ���________���ȵķ�����
���𰸡�B 1:2 g,f��d,e��b,c��h Cl2����ˮ���������¿��淴Ӧ��![]() ���ڱ���ʳ��ˮ�У�c(Cl-)�ϴ�ʹƽ�����ƣ������ܽ�ȼ�С�����Ȼ������弫������ˮ������ ������ƿ ���ܼ�������Ӧ��ʹ�����ĽӴ������ʹ���ַ�Ӧ ��ֹ���Ȼ���ˮ�����ˮ��������ȩ ˮԡ
���ڱ���ʳ��ˮ�У�c(Cl-)�ϴ�ʹƽ�����ƣ������ܽ�ȼ�С�����Ȼ������弫������ˮ������ ������ƿ ���ܼ�������Ӧ��ʹ�����ĽӴ������ʹ���ַ�Ӧ ��ֹ���Ȼ���ˮ�����ˮ��������ȩ ˮԡ
��������
ʵ�����Ʊ�����Ϊ�������̺�Ũ���ᷴӦ���������ȣ����շ������������ڼ��ȷ�Ӧ�����ռ����������У�Ҫ���ñ���ʳ��ˮ���ջӷ����Ȼ��⣬Ȼ����Ũ������������ſ������ռ���β���ü�ʯ�����ա�
��1��ʵ����Cl2���Ʊ�ѡ�ö������̺�Ũ������м��ȣ����ڹ�Һ������װ�ã��÷�������ѡ��ͼ�е�BΪCl2����װ�ã���Ӧ��������Ϊ�������̣���ԭ��Ϊ���ᣬ��ӦΪMnO2 + 4H+ + 2Cl- = Mn2+ + Cl2��+ 2H2O�����ʵ���֮��Ϊ1:2��
��2��ѡ��ͼ�е�װ���ռ�һƿ���������Cl2��Ũ���������ˮ�ԣ�����ʳ��ˮ���ջӷ������Ȼ�������ͽ����������ܽ�ȣ���ʯ�ҷ�ֹ����й©������β�����������ӿڵ�����˳��Ϊa��g,f��d,e��b,c��h��
��3���ñ���ʳ��ˮ��ȥCl2�л��е�HCl��ԭ��ΪCl2����ˮ���������¿��淴Ӧ��![]() ���ڱ���ʳ��ˮ�У�c(Cl-)�ϴ�ʹƽ�����ƣ������ܽ�ȼ�С�����Ȼ������弫������ˮ�����ա�
���ڱ���ʳ��ˮ�У�c(Cl-)�ϴ�ʹƽ�����ƣ������ܽ�ȼ�С�����Ȼ������弫������ˮ�����ա�
��4��ͼ������A��������������ƿ��
��5�����ȱ���ȩ���Ʊ������У�������������������ܼ�������Ӧ��ʹ�����ĽӴ������ʹ���ַ�Ӧ����ʵ��Ҫ����ˮ�����������Ƿ�ֹ���Ȼ���ˮ�����ˮ��������ȩ����ʵ�������κ��¹��̣�Ϊ���Ʒ�Ӧ�¶�����A���ɲ���ˮԡ���ȵķ�����


 �������¿��ÿ�ʱ��ҵϵ�д�
�������¿��ÿ�ʱ��ҵϵ�д� Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ������ȤС��Ϊ��֤±�ص��������Ե����ǿ��������ͼ��ʾװ�ý���ʵ��(�г���������ȥ���������Ѽ���)��
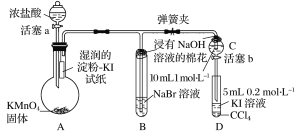
ʵ����̣�
��.���ɼУ�����a���μ�Ũ���ᣬ������Ӧ2KMnO4��16HCl(Ũ)��2KCl��2MnCl2��5Cl2����8H2O��
��.��B��C�е���Һ����Ϊ��ɫʱ���н����ɼС�
��.��B����Һ�ɻ�ɫ��Ϊ����ɫʱ���رջ���a��
��.����
(1)ʢ��Ũ�������������Ϊ____________________��
(2)����ƿ�ڷ�����Ӧʱ������Ũ���������Ϊ______________________________��
(3)��֤������������ǿ�ڵ��ʵ��������_________________________________��
(4)B����Һ������Ӧ�����ӷ���ʽ��____________________________________________��
(5)����NaOH��Һ����������Ϊ______________________________________________��
(6)Ϊ��֤���������ǿ�ڵ⣬�������IJ��������������__________________________��
(7)������ʵ���Ŀ����______________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ�ʮ�����ӱ�������������Ⱦ������������ƣ��������µ�Ŀ������·�ϡ�����̼�Ĵ���������ﶼ�ǿ�����Ⱦ����������գ�
I.��1��̼ԭ�ӵ����������Ų�ʽΪ___����ԭ�Ӻ���������ߵ���Щ����֮����Ƚϣ����Dz���ͬ���˶�״̬Ϊ___����Ԫ�صķǽ����Ա�̼Ԫ��ǿ����֤���ý��۵��ǣ�ѡ���ţ�___��
A.���ǵ���̬�⻯����ȶ���
B.������Ԫ�����ڱ��е�λ��
C.�����֮���γɵĻ�������Ԫ�صĻ��ϼ�
D.���ǵ�����������Ӧˮ���������ǿ��
��.��֪NO2(g)+SO2(g)![]() NO(g)+SO3(g)����һ���ݻ����ܱ������н��и÷�Ӧ��
NO(g)+SO3(g)����һ���ݻ����ܱ������н��и÷�Ӧ��
��2����һ�������£�������ѹǿ�������仯ʱ��___����ܡ����ܡ���˵���÷�Ӧ�Ѿ��ﵽ��ѧƽ��״̬�������ǣ�___��
��һ���¶��£����ӷ�Ӧ��ϵ�з����SO3������ƽ���ƶ������У�ѡ���ţ�___��
A.Kֵ��С B.�淴Ӧ�����ȼ�С������
C.Kֵ���� D.����Ӧ���ʼ�С�������
��.��ѧ���о����ô������������·�Ӧ��2NO2+4CO![]() N2+4CO2+Q(Q��0)
N2+4CO2+Q(Q��0)
��3��д���÷�Ӧ��ϵ�����ڷǼ��Է����ҹ��õ��Ӷ������ٵ����ʵĵ���ʽ___�����÷�Ӧ����������ۣ���Ӧ�������ԣ�___��___��
���÷�Ӧ���������Ũ����2min�ڼ�����0.2mol/L������NO2����ʾ��Ӧ�ڴ�2min�ڵ�ƽ������Ϊ___��
��4����֪ѹǿP2��P1������ͼ�������÷�Ӧ��P2�����µı仯����___��

�÷�Ӧ�Ծ��������������á���˵���÷�Ӧ����Ҫѡ��һ�����˵��¶Ƚ��е�ԭ���ǣ�___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������������Ʊ�����һ֧�Թ��м�������������������Һ��Ȼ���������������Һ��
(1)�۲쵽��ʵ��������_________��
(2)�û�ѧ����ʽ��ʾ�������������ԭ��_______________��________________
(3)����ȡ����������ʱ���ɽ���������������Һ�ij���ͷ�ι����뵽��������Һ���£��ټ�������������Һ��Ŀ����_________��
(4)���и�ͼ��ʾ��ʵ�����ܽϳ�ʱ�俴��Fe(OH) 2 ��ɫ��������_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������������ɴ�����Ⱦ����Ҫ���ʡ��о����ǵķ�Ӧ��������������������Ⱦ����Ҫ���塣
��1��2NO(g)+O2(g)=2NO2(g)��H�ķ�Ӧ��������Ԫ��Ӧ����Ԫ��Ӧ��ָ�ڷ�Ӧ��һ��ֱ��ת��Ϊ����ķ�Ӧ���ֳ�Ϊ��Ӧ���Ļ��Ϊ��
2NO(g)![]() N2O2(g)E1=82kJ��mol��1v=k1c2(NO)
N2O2(g)E1=82kJ��mol��1v=k1c2(NO)
N2O2(g)![]() 2NO(g)E-1=205kJ��mol��1v=k��1c(N2O2)
2NO(g)E-1=205kJ��mol��1v=k��1c(N2O2)
N2O2(g)��O2(g)![]() 2NO2(g)E2=82kJ��mol��1v=k2c(N2O2)��c(O2)
2NO2(g)E2=82kJ��mol��1v=k2c(N2O2)��c(O2)
2NO2(g)![]() N2O2(g)��O2(g)E��2=72kJ��mol��1v=k��2c2(NO2)
N2O2(g)��O2(g)E��2=72kJ��mol��1v=k��2c2(NO2)
��2NO(g)+O2(g)![]() 2NO2(g)��H=______kJmol-1��ƽ�ⳣ��K��������Ӧ���ʳ���k1��k-1��k2��k-2�Ĺ�ϵʽΪK=_______��
2NO2(g)��H=______kJmol-1��ƽ�ⳣ��K��������Ӧ���ʳ���k1��k-1��k2��k-2�Ĺ�ϵʽΪK=_______��
��ij�¶��·�Ӧ2NO(g)+O2(g)![]() 2NO2(g)�����ʳ���k=8.8��10-2L2mol-2s-1������Ӧ��Ũ�ȶ���0.05molL-1ʱ����Ӧ��������__________________molL-1s-1������ʱ��С�����������ʹ����ѹǿ����Ϊԭ����2������Ӧ��������Ϊ֮ǰ��______����
2NO2(g)�����ʳ���k=8.8��10-2L2mol-2s-1������Ӧ��Ũ�ȶ���0.05molL-1ʱ����Ӧ��������__________________molL-1s-1������ʱ��С�����������ʹ����ѹǿ����Ϊԭ����2������Ӧ��������Ϊ֮ǰ��______����
��2��2SO2(g)+O2(g)![]() 2SO3(g)��Ӧ�����������仯��ͼ1��ʾ����V2O5����ʱ���÷�Ӧ�Ļ���Ϊ��V2O5+SO2
2SO3(g)��Ӧ�����������仯��ͼ1��ʾ����V2O5����ʱ���÷�Ӧ�Ļ���Ϊ��V2O5+SO2![]() 2VO2+SO3(��)4VO2+O2
2VO2+SO3(��)4VO2+O2![]() 2V2O5(��)
2V2O5(��)
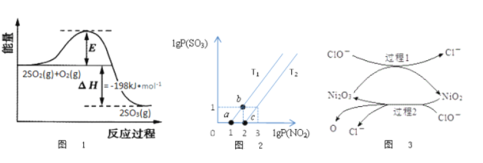
����˵����ȷ����___________
A����Ӧ������Ҫȡ����V2O5������
B��VO2�Ǹ÷�Ӧ�Ĵ���
C���淴Ӧ�Ļ�ܴ���198kJ/mol
D������SO2��Ũ�ȿ�������߷�Ӧ����
��3��ij�о�С���о�T1�桢T2��ʱ��������������ת����NO2(g)+SO2(g)![]() NO(g)+SO3(g)��lgP(NO2)��lgP(SO3)��ϵ��ͼ2��ʾ��ʵ���ʼʱ��ϵ�е�P(NO2)��P(SO2)��ȡ�P(NO)��P(SO3)��ȡ�
NO(g)+SO3(g)��lgP(NO2)��lgP(SO3)��ϵ��ͼ2��ʾ��ʵ���ʼʱ��ϵ�е�P(NO2)��P(SO2)��ȡ�P(NO)��P(SO3)��ȡ�
�ٸ��������֪��T1______T2����������������ߡ�=����,������___________________��
����ƽ��״̬a��b���ı��������__________________��
��4����ҵ�Ͽ���NaClO������Һ���������η�������SO2��Ϊ���������Ч�ʣ�����Ni2O3��Ϊ��������������ͼ3��ʾ��
�ٹ���2�����ӷ���ʽ_______________________________��
��Ca(C1O)2Ҳ����������������Ч����NaC1O���ã�ԭ����________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�0.1 mol��L��1��HA��Һ��c(OH��)/c(H��)��1��10��8��������������ȷ����(����)
A. 0.01 mol��L��1HA����Һ��c(H��)��1��10��4mol��L��1
B. pH��3��HA��Һ��pH��11��NaOH��Һ�������Ϻ�������Һ��c(Na��)��c(A��)��c(OH��)��c(H��)
C. Ũ�Ⱦ�Ϊ0.1 mol��L��1��HA��Һ��NaA��Һ�������Ϻ�������Һ�����ԣ���c(OH��)��c(H��)��c(HA)��c(A��)
D. pH��3��HA��Һ��pH��11��NaOH��Һ�������1��10��Ϻ�������Һ��c(OH��)��c(A��)��c(H��)��c(Na��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ���������Ҫ��ӦΪ4NH3(g)+5O2(g)4NO(g)+6H2O(l)��H
(1)��֪��������ȼ����Ϊ285.8kJmol-1
N2(g)+3H2(g)2NH3(g)��H=-92.4kJmol-1
N2(g)+O2(g)2NO(g)��H=+180.6kJmol-1
��������ҵ���������Ҫ��Ӧ����H=______��
(2)���ݻ��̶����ܱ������з���������Ӧ�������ڲ������ʵ����ʵ���Ũ�����±���
Ũ�� | c(NH3)(molL-1) | c(O2)(molL-1) | c(NO)(molL-1) |
��ʼ | 0.8 | 1.6 | 0 |
��2min | 0.6 | a | 0.2 |
��4min | 0.3 | 0.975 | 0.5 |
��6min | 0.3 | 0.975 | 0.5 |
��8min | 0.7 | 1.475 | 0.1 |
�ٷ�Ӧ�ڵ�2min����4min�ڣ�O2��ƽ����Ӧ����Ϊ______��
�ڷ�Ӧ�ڵ�6minʱ�ı����������ı������������______(�����)��
A ʹ�ô���������B �����¶�C ��СѹǿD ����O2��Ũ��
������˵������˵��4NH3(g)+5O2(g)4NO(g)+6H2O(g)�ﵽƽ��״̬����_____(�����)��
A ��λʱ��������nmolNO��ͬʱ������nmolNH3
B ����һ������������ƽ����Է����������ٱ仯
C �ٷֺ���w(NH3)=w(NO)
D ��Ӧ����v(NH3)��v(O2) ��v(NO) ��v(H2O)=4��5��4��6
E ���ں��º�ѹ���ݻ��ɱ�������з�Ӧ�����������ܶȲ��ٱ仯
(3)ij�о�����װ��CH3OH-O2ȼ�ϵ�صĹ���ԭ����ͼ��ʾ��
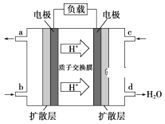
�ٸõ�ع���ʱ��b��ͨ�������Ϊ______��
�ڸõ�������ĵ缫��ӦʽΪ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ��ȤС��Ϊ̽��Ԫ�����ʵĵݱ���ɣ����������ϵ��ʵ�顣
��.��1�����ơ��ء�þ������1 mol�ֱ�Ͷ�뵽������0.1 mol��L��1�������У���Ԥ��ʵ������__________�����ᷴӦ����ң�__________�����ᷴӦ������
��2����NaOH��Һ��NH4Cl��Һ�������NH3��H2O���Ӷ���֤NaOH�ļ��Դ���NH3��H2O���̶�������֤Na�Ľ����Դ���N������Ϊ������Ƿ��������˵�����ɣ�_______,________________��
��.������ͼװ�ÿ�����֤�ǽ����Եı仯���ɡ�

��3������A������Ϊ________�������D��������_____________________________��
��4��ʵ����������ҩƷNa2S��KMnO4��Ũ���ᡢMnO2����ѡ�����ҩƷ���ʵ����֤�ȵķǽ����Դ�����װ��A��B��C����װҩƷ�ֱ�Ϊ________��________��________��װ��C�е�ʵ������Ϊ�е���ɫ�������ɣ����ӷ���ʽΪ_________________________��
��5����Ҫ֤���ǽ����ԣ�C>Si����A�м�________��B�м�Na2CO3��C�м�________���۲쵽C����Һ������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij���Ϳɳ���ع�����ͼ��ʾ������ʱ(��������Fe ��KClO3�ķ�Ӧ���Ӷ�ʹ LiCl-KCl�������ۻ�)��ij�缫(��ΪX )�ķ�Ӧʽ֮һΪ��xLi++ xe- +LiV3O8=Lii+xV3O8������˵����ȷ����

A.�ŵ�ʱ�������ĵ缫��ӦʽΪ: Li �C e- = Li+
B.�ŵ�ʱ���ܷ�ӦʽΪ��xLi + LiV3O8 = Lii+xV3O8
C.���ʱ��X�缫����ӵ�Դ��������
D.���ʱ��X�缫����������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com