
�������ֿ���������A��B��C��
D��E�������������������ӻ�����ͬ���ֱ�������������Na����Al3����Mg2����Ba2����Fe3��������������Cl����OH����NO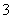 ��CO
��CO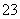 ��X�е�һ�֡�
��X�е�һ�֡�
(1)ijͬѧͨ���ȽϷ�������Ϊ�������Ϳ��ж����б��е�����������________��________(�ѧʽ)��
(2)Ϊ��ȷ��X���ֽ�(1)�е��������ʼ�ΪA��B����X�����ʼ�ΪC����C��B����Һ���ʱ���������ɫ��������ɫ��ζ���壻��C��A����Һ���ʱ�����ػ�ɫ��������ó����е���ϡ������������ܽ⣬������а�ɫ���������ܽ⡣��XΪ________(��ѡ����ĸ)��
A��SO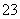 ���������������� B��SO
���������������� B��SO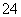
C��CH3COO�� D��SiO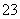
(3)��CuͶ�뵽װ��D��Һ���Թ��У�Cu���ܽ⣻�ٵμ�ϡH2SO4��Cu���ܽ⣬�ܿڸ����к���ɫ������֡�������Dһ���������������е�________(����Ӧ�����ӷ���)���йط�Ӧ�����ӷ���ʽΪ_____________��
(4)���������Ѿ�ȷ�������ʣ����Լ����D��E�е������ӡ������ʵ��������衢������_______________________________________________________________________________________________________________________________________________________��
������(1)����̼�����У�ֻ��Na2CO3�ǿ��ܵģ����ּ��У�ֻ��NaOH��Ba(OH)2�ǿ��ܵģ���Ϊ�������������������ӻ�����ͬ�������������ʷֱ���Na2CO3��Ba(OH)2��
(2)����ʵ�������֪CΪFe2(SO4)3��Fe2(SO4)3��Na2CO3����˫ˮ�ⷴӦ�����������������Ͷ�����̼�������ɣ�Fe2(SO4)3��Ba(OH)2��Ӧ�������������������ᱵ�������ɣ�����ϡ���ᣬֻ���������������ܽ⡣
(3)����ʵ������Cu��NO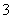 �����������·�����Ӧ��
�����������·�����Ӧ�� 3Cu��8H����2NO
3Cu��8H����2NO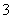 ===3Cu2����2NO����4H2O��
===3Cu2����2NO����4H2O��
(4)����������������ǿ������ʽ��м��顣
�𰸡�(1)Na2CO3��Ba(OH)2
(2)B
(3)NO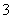 ��3Cu��8H����2NO
��3Cu��8H����2NO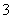 ===3Cu2����2NO����4H2O
===3Cu2����2NO����4H2O
(4)��D����Һ����μ���Ba(OH)2��Һֱ�����������ȳ��ְ�ɫ�����������ܽ⣬��D�к���Al3���������ɵİ�ɫ�������ܽ⣬��D�к���Mg2��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�ʵ���������ȷ���ǣ� ��
A����һ����������ͨ��30 mLŨ��Ϊ10.0mol/L����������Ũ��Һ�У���������ʱ�����Һ���γ�NaCl��NaClO��NaClO3������ϵ��n(NaCl)��n(NaClO)��n(NaClO3)����Ϊ11��2��1
B��ʵ���ҿ�������һ�ֽ���Al3+��K+��SO42-��NO3-��4�����ӣ���������Դ��ˮ��������ӣ�����Һ����4�����ӵ�Ũ�Ⱦ�Ϊ1mol/L
C��HCl��FeCl3��Fe3O4��NaOH����ͨ���û���Ӧһ���õ�Ҳ��ͨ�����Ϸ�Ӧһ���õ�
D��ʵ������������Ϊ�˼ӿ췴Ӧ���ʣ�����ϡH2SO4�еμ�����Cu(NO3)2��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��4 g NaOH����ˮ���50 mL��Һ��ȡ��5 mL����5 mL��Һ�����ʵ���Ũ���� ( )
A��2 mol/L B��1 mol/L C��0.1 mol/L D��0.05 mol/
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����(����)
��ά����C���л�ԭ�ԣ�������������������
��NO2����ˮʱ����������ԭ��Ӧ����1 mol Cl2�μӷ�Ӧת�Ƶ�����һ��Ϊ2NA���������Ӷ�ֻ�л�ԭ��
A���٢� B���ڢ�
C���ۢ� D���٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£����и���������ָ����Һ���ܴ����������(����)
A��pH ��1����Һ�У�K����Fe2����MnO
��1����Һ�У�K����Fe2����MnO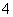 ��SO
��SO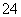
B��c(Fe3��)��0.1 mol��L��1����Һ�У�K����ClO����SO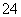 ��SCN��
��SCN��
C��c(H��)/c(OH��)��1012����Һ�У�NH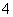 ��Al3��
��Al3�� ��NO
��NO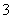 ��Cl��
��Cl��
D��������Ӧ������������Һ�У�NH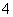 ��K����Cl����SiO
��K����Cl����SiO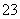
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ��д��ȷ����(����)
A����Pt�缫���������MgCl2��Һ��2H2O��2Cl��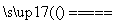 H2����Cl2����2OH��
H2����Cl2����2OH��
B���������������м���������ϡ���Fe(OH)2��2H��===Fe2����2H2O
C����NaAlO2��Һ��ͨ�����CO2��Al(OH)3��AlO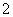 ��CO2��2H2O===Al(OH)3����HCO
��CO2��2H2O===Al(OH)3����HCO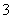
D����ʳ�׳�ȥˮƿ�е�ˮ����CO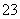 ��2CH3COOH===2CH3COO����CO2����H2O
��2CH3COOH===2CH3COO����CO2����H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ҵ��ˮ�����±��е�ijЩ���ӣ��Ҹ������ӵ����ʵ���Ũ����ȣ���Ϊ0.1 mol/L(����ֵ����ˮ�ĵ��뼰���ӵ�ˮ��)��
| ������ | K�� | Ag�� | Mg2�� | Cu2�� | Al3�� | NH |
| ������ | Cl�� | CO | NO | SO | SiO | I�� |
��ͬѧ��̽����ˮ����ɣ�����������ʵ�飺
��.ȡ����ɫ��Һ5 mL���μ�һ�ΰ�ˮ�г������ɣ��������������ӡ�
��.�ò�˿պȡ��Һ���ڻ��������գ�����ɫ�ܲ����۲죬����ɫ���档
��.��ȡ��Һ����������ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ��
��.��������õ���Һ�м���BaCl2��Һ���а�ɫ�������ɡ�
���ƶϣ�
(1)�ɢ��жϣ���Һ��һ�������е���������______��
( 2)���м�������������ɫ��������ӷ���ʽ��________________��
2)���м�������������ɫ��������ӷ���ʽ��________________��
(3)��ͬѧ����ȷ��ԭ��Һ��������������________����������________�����ݴ��Ʋ�ԭ��ҺӦ�ó�________�ԣ�ԭ����________________________(�������ӷ���ʽ˵��)��
(4)��ȡ100 mLԭ��Һ������������NaOH��Һ���˹������漰�����ӷ���ʽΪ_____________________________________________________
___________________________________________________________��
��ַ�Ӧ����ˣ�ϴ�ӣ����ճ��������أ��õ��Ĺ�������Ϊ________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��CaCO3(s)===CaO��CO2(g)����H��177.7 kJ
��C(s)��H2O(s)===CO(g)��H2(g)
��H����131.3 kJ/mol
��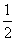 H2SO4(l)��NaOH(l)===
H2SO4(l)��NaOH(l)===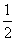 Na2SO4(l)��H2O(l)
Na2SO4(l)��H2O(l)
��H����57.3 kJ/mo l
l
��C(s)��O2(g)===CO2(g)����H����393.5 kJ/mol
��CO(g)��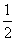 O2(g)===CO2(g)����H����283 kJ/mol
O2(g)===CO2(g)����H����283 kJ/mol
��HNO3(aq)��NaOH(aq)===NaNO3(aq)��H2O(l)
��H����57.3 kJ/mol
��2H2(g)��O2(g)===2H2O(l)
��H����517.6 kJ/mol
(1)�����Ȼ�ѧ����ʽ�У�����ȷ����________������ȷ�����ɷֱ���______________________________________________________________________________________________________________________________��
(2)����������Ϣ��д��Cת��ΪCO���Ȼ�ѧ����ʽ
_________________________________________________________��
(3)������Ӧ�У���ʾȼ���ȵ��Ȼ�ѧ����ʽ��______________����ʾ�к��ȵ��Ȼ�ѧ����ʽ��__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������С����������˷�������ˮ�ȣ��������ʿ����ڼ��ᶾ�Ե���(����)
A����ˮ������������������ B���ƾ�
C����ˮ D��ʳ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com