
£Ø14·Ö£©¢ń.ĻĀĮŠŹµŃé²Ł×÷»ņ¶ŌŹµŃéŹĀŹµµÄĆčŹöÕżČ·µÄŹĒ £ØĢīŠņŗÅ£©
¢Ł ŹµŃéŹŅÅäÖĘĀČ»ÆŃĒĢśČÜŅŗŹ±£¬½«ĀČ»ÆŃĒĢśĻČČܽāŌŚŃĪĖįÖŠ£¬Č»ŗóÓĆÕōĮóĖ®Ļ”ŹĶ²¢¼ÓČėÉŁĮæĢś·Ū£»
¢Ś ÅäÖĘŅ»¶ØÅØ¶ČµÄČÜŅŗŹ±£¬ø©ŹÓČŻĮæĘæµÄæĢ¶ČĻߣ¬»įŹ¹ÅäÖʵÄÅضČĘ«øߣ»ŹµŃéŹŅ²ā¶ØÖŠŗĶČČŹ±£¬¹żŌē¶ĮŹż»įŹ¹²ā¶Ø½į¹ūĘ«µĶ£»
¢Ū ŹŌ¹ÜÖŠ¼ÓČėÉŁĮæµķ·Ū£¬ŌŁ¼ÓČėŅ»¶ØĮæĻ”ĮņĖį£¬¼ÓČČ3£4·ÖÖÓ£¬Č»ŗó¼ÓČėŅų°±ČÜŅŗ£¬Ę¬æĢŗó¹Ü±ŚÉĻÓŠ”°Ņų¾µ”±³öĻÖ
¢Ü ·Ö±šĻņĢå»żŗĶpH¾łĻąĶ¬µÄŃĪĖįŗĶ“×ĖįÖŠµĪ¼ÓµČÅØ¶ČµÄĒāŃõ»ÆÄĘČÜŅŗ£¬ĶźČ«ÖŠŗĶŹ±ĻūŗĵÄĒāŃõ»ÆÄĘČÜŅŗµÄĢå»żŅ»Ńł¶ą
¢Ż ĻņNaOHČÜŅŗ”¢KSCNČÜŅŗ”¢·ŠĢŚµÄÕōĮóĖ®ÖŠ·Ö±šµĪ¼Ó±„ŗĶµÄFeCl3ČÜŅŗµĆµ½µÄ·ÖÉ¢ĻµŅĄ“ĪĪŖ£ŗ×ĒŅŗ”¢ČÜŅŗ”¢½ŗĢå
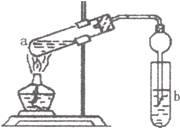
¢ņ.Ä³ŃŠ¾æŠŌѧĻ°Š”×éĄūÓĆĻĀĶ¼ĖłŹ¾×°ÖĆŃŠ¾æŅŅ“¼ÓėŃõ»ÆĢśµÄ·“Ó¦£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©×°ÖĆÖŠŹŌ¹ÜBµÄ×÷ÓĆŹĒ ”£
£Ø2£©ŹµŃéÖŠæɹŪ²ģµ½ŹÆÓ¢¹ÜAÖŠµÄĻÖĻóĪŖ ”£
£Ø3£©·“Ó¦Ķ£Ö¹ŗó£¬Č”³öŹŌ¹ÜCŌŚ¾Ę¾«µĘÉĻ¼ÓČČÖĮ·ŠĢŚ£¬æɹŪ²ģµ½ÓŠŗģÉ«³Įµķ²śÉś”£Š“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
£Ø4£©ĪŖĮĖ²ā¶Ø·“Ó¦ŗóŹÆÓ¢¹ÜA×ó²ą¹ĢĢåÖŠĢśŌŖĖŲµÄŗ¬Į棬½ųŠŠČēĻĀŹµŃé£ŗ
£Øi£©²½Öč¢ŪÖŠÓƵ½µÄ²£Į§ŅĒĘ÷ÓŠÉÕ±”¢²£Į§°ō”¢½ŗĶ·µĪ¹Ü”¢ ”£
£Øii£©ĻĀĮŠÓŠ¹Ų²½Öč¢ÜµÄ²Ł×÷ÖŠĖµ·ØÕżČ·µÄŹĒ ”£
a£®µĪ¶Ø¹ż³ĢÖŠæÉĄūÓƵķ·ŪČÜŅŗ×÷ĪŖÖøŹ¾¼Į
b£®µĪ¶Ø¹ÜÓĆÕōĮóĖ®Ļ“µÓŗóæÉŅŌÖ±½Ó×°Ņŗ
c£®×¶ŠĪĘæ²»ŠčŅŖÓĆ“ż²āŅ¹ČóĻ“
d£®µĪ¶Ø¹ż³ĢÖŠ£¬ŃŪ¾¦×¢ŹÓµĪ¶Ø¹ÜÖŠŅŗĆę±ä»Æ
e£®µĪ¶Ø½įŹųŗó£¬30 sÄŚČÜŅŗ²»»Öø“ŌĄ“µÄŃÕÉ«£¬ŌŁ¶ĮŹż
£Øiii£©ÓÉæņĶ¼ÖŠŹż¾Ż¼ĘĖć£¬æɵƏÆÓ¢¹ÜA×ó²ą¹ĢĢåÖŠĢśŌŖĖŲµÄ°Ł·Öŗ¬ĮæĪŖ ”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 £Ø1£©ĻĀĮŠŹµŃé²Ł×÷»ņ¶ŌŹµŃéŹĀŹµµÄŠšŹöÕżČ·µÄŹĒ
£Ø1£©ĻĀĮŠŹµŃé²Ł×÷»ņ¶ŌŹµŃéŹĀŹµµÄŠšŹöÕżČ·µÄŹĒ²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com