
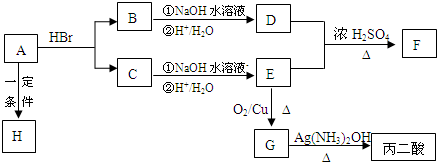
��һ��������A��B��C��D��E���ֶ�����Ԫ�ص�ijЩЩ����
|
| A | B | C | D | E |
| ���ϼ� | ��4 | ��2 | ��1 | ��2 | ��1 |
| �縺�� | 2.5 | 2.5 | 3.0 | 3.5 | 4.0 |
(1)Ԫ��A���γ��л������ҪԪ�أ����з����к���sp��sp3 �ӻ���ʽ����
A��![]() B��CH4 C��CH2��CHCH3 D��CH3CH2C��CH E��CH3CH3
B��CH4 C��CH2��CHCH3 D��CH3CH2C��CH E��CH3CH3
(2)��AD2��Ϊ�ȵ�����ķ��ӡ����ӵĻ�ѧʽ����Ϊ �� ����д1�֣�
(3)��ͬ�����£�AD2��BD2����������ˮ�е��ܽ�Ƚϴ���� ��д����ʽ���������� ��
�����������Dz��ֽ���Ԫ�صĵ�����
| X | Y | Z | |
| ��һ������(kJ/mol) | 520.2 | 495.8 | 418.8 |
 ��4����֪X��Y��Z�ļ۲���ӹ���Ϊns1�������ֽ������Ȼ��RCl�����۵��ɵ͵��ߵ�˳��Ϊ ��
��4����֪X��Y��Z�ļ۲���ӹ���Ϊns1�������ֽ������Ȼ��RCl�����۵��ɵ͵��ߵ�˳��Ϊ ��
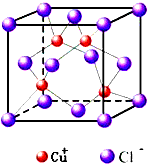
��5��CuCl�����л��ϳɴ���, ����������, �����ȹ�ҵ��CuCl�ľ���ṹ��ͼ��ʾ��Ԫ��Cu��̬ԭ�ӵĵ����Ų�ʽ ����ͬһ��Cl�������� Cu+�� ����
 �������¿��ÿ�ʱ��ҵϵ�д�
�������¿��ÿ�ʱ��ҵϵ�д� Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
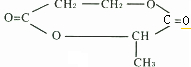
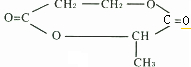





�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
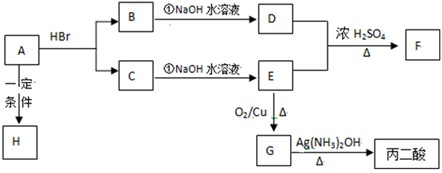
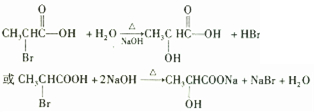
��Ŀ�����л�ѧ ��Դ��0110 ģ���� ���ͣ������

 B��CH4 C��CH2��CHCH3 D��CH3CH2C��CH E��CH3CH3
B��CH4 C��CH2��CHCH3 D��CH3CH2C��CH E��CH3CH3

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ģ���� ���ͣ������
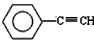
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com